Lack of Concordance in Symptomatic Adverse Event Reporting by Children, Clinicians, and Caregivers: Implications for Cancer Clinical Trials
- PMID: 35294262
- PMCID: PMC9113216
- DOI: 10.1200/JCO.21.02669
Lack of Concordance in Symptomatic Adverse Event Reporting by Children, Clinicians, and Caregivers: Implications for Cancer Clinical Trials
Abstract
Purpose: To examine concordance in symptomatic adverse event (AE) grading using the Common Terminology Criteria for Adverse Events (CTCAE 4.0) for clinicians and its patient-reported outcome (PRO) versions for children (Ped-PRO-CTCAE) and caregivers (Ped-PRO-CTCAE [Caregiver]).
Methods: Children age 7-18 years with a first cancer diagnosis, their clinicians, and caregivers completed CTCAE-based measures before starting a treatment course (T1) and after the treatment (T2). Grades (0-3) were assigned by each reporter for 15 core AEs spanning physical and mental health. Mean grades were compared between reporters using two-sample t-tests; agreement was estimated using weighted kappa (κ) statistics. Multivariable mixed regression models were used to evaluate associations of clinical factors with AE reporting concordance. Significance was set at α = .05 (two-sided).
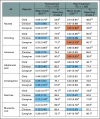
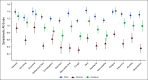
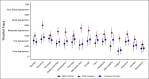
Results: There were 438 child-clinician-caregiver triads with complete data at either T1 or T2. For children, the mean age was 13 years (standard deviation = 3.4), 53.7% were male, 32.6% non-White, and 56.4% had leukemia/lymphoma. At T1, clinician mean AE grades were significantly lower (ie, better) than children for all AEs and remained significantly lower at T2 except for constipation, nausea, anorexia, neuropathy, and anxiety. Caregiver mean AE grades were similar to children at T1 and significantly higher (ie, worse) at T2 for nausea, vomiting, anorexia, pain, fatigue, anxiety, and depression. Agreement for child-clinician grading was poor-to-fair at T1 (κ range, 0.08-0.34) and T2 (0.11-0.35), and for child-caregiver, was fair-to-good at T1 (0.34-0.65) and T2 (0.24-0.60). No factors were consistently associated with reporter concordance across AEs.
Conclusion: Compared with children, symptomatic AEs were consistently under-reported by clinicians with low agreement and over-reported by caregivers with low-moderate agreement. Direct reporting by children using Ped-PRO-CTCAE or similar measures should be routinely incorporated for toxicity assessment in clinical trials.
Conflict of interest statement
Figures





Comment in
-
We Cannot Change What We Cannot See: A Rationale for Patient-Reported Outcomes in Pediatric Oncology Clinical Research.J Clin Oncol. 2022 May 20;40(15):1601-1603. doi: 10.1200/JCO.22.00196. Epub 2022 Mar 16. J Clin Oncol. 2022. PMID: 35294267 No abstract available.
References
-
- Number of Diagnoses, CureSearch for Children's Cancer. 2020. https://curesearch.org/number-of-diagnoses
-
- Sateren WB, Trimble EL, Abrams J, et al. : How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol 20:2109-2117, 2002 - PubMed
-
- Bleyer A, Budd T, Montello M: Adolescents and young adults with cancer: The scope of the problem and criticality of clinical trials. Cancer 107:1645-1655, 2006 - PubMed
-
- NCI Guidelines for Investigators: Adverse Event Reporting Requirements for DCTD (CTEP and CIP) and DCP INDs and IDEs. 2013. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs...
-
- Sivendran S, Latif A, McBride RB, et al. : Adverse event reporting in cancer clinical trial publications. J Clin Oncol 32:83-89, 2014 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

