Finger stability in precision grips
- PMID: 35294291
- PMCID: PMC8944252
- DOI: 10.1073/pnas.2122903119
Finger stability in precision grips
Abstract
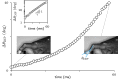
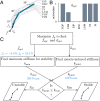
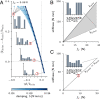
Stable precision grips using the fingertips are a cornerstone of human hand dexterity. However, our fingers become unstable sometimes and snap into a hyperextended posture. This is because multilink mechanisms like our fingers can buckle under tip forces. Suppressing this instability is crucial for hand dexterity, but how the neuromuscular system does so is unknown. Here we show that people rely on the stiffness from muscle contraction for finger stability. We measured buckling time constants of 50 ms or less during maximal force application with the index finger—quicker than feedback latencies—which suggests that muscle-induced stiffness may underlie stability. However, a biomechanical model of the finger predicts that muscle-induced stiffness cannot stabilize at maximal force unless we add springs to stiffen the joints or people reduce their force to enable cocontraction. We tested this prediction in 38 volunteers. Upon adding stiffness, maximal force increased by 34 ± 3%, and muscle electromyography readings were 21 ± 3% higher for the finger flexors (mean ± SE). Muscle recordings and mathematical modeling show that adding stiffness offloads the demand for muscle cocontraction, thus freeing up muscle capacity for fingertip force. Hence, people refrain from applying truly maximal force unless an external stabilizing stiffness allows their muscles to apply higher force without losing stability. But more stiffness is not always better. Stiff fingers would affect the ability to adapt passively to complex object geometries and precisely regulate force. Thus, our results show how hand function arises from neurally tuned muscle stiffness that balances finger stability with compliance.
Keywords: cocontraction; human hand; muscle elasticity; precision grip; stability.
Conflict of interest statement
The authors declare no competing interest.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

