Differential viral RNA methylation contributes to pathogen blocking in Wolbachia-colonized arthropods
- PMID: 35294495
- PMCID: PMC8959158
- DOI: 10.1371/journal.ppat.1010393
Differential viral RNA methylation contributes to pathogen blocking in Wolbachia-colonized arthropods
Abstract
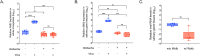
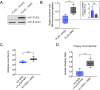
Arthropod endosymbiont Wolbachia pipientis is part of a global biocontrol strategy to reduce the replication of mosquito-borne RNA viruses such as alphaviruses. We previously demonstrated the importance of a host cytosine methyltransferase, DNMT2, in Drosophila and viral RNA as a cellular target during pathogen-blocking. Here we report a role for DNMT2 in Wolbachia-induced alphavirus inhibition in Aedes species. Expression of DNMT2 in mosquito tissues, including the salivary glands, is elevated upon virus infection. Notably, this is suppressed in Wolbachia-colonized animals, coincident with reduced virus replication and decreased infectivity of progeny virus. Ectopic expression of DNMT2 in cultured Aedes cells is proviral, increasing progeny virus infectivity, and this effect of DNMT2 on virus replication and infectivity is dependent on its methyltransferase activity. Finally, examining the effects of Wolbachia on modifications of viral RNA by LC-MS show a decrease in the amount of 5-methylcytosine modification consistent with the down-regulation of DNMT2 in Wolbachia colonized mosquito cells and animals. Collectively, our findings support the conclusion that disruption of 5-methylcytosine modification of viral RNA is a vital mechanism operative in pathogen blocking. These data also emphasize the essential role of epitranscriptomic modifications in regulating fundamental alphavirus replication and transmission processes.
Conflict of interest statement
The authors have declared no competing interests exist.
Figures




References
-
- Díaz-Sánchez S, Hernández-Jarguín A, Torina A, Fernández de Mera I, Estrada-Peña A, Villar M, et al.. Biotic and abiotic factors shape the microbiota of wild-caught populations of the arbovirus vector Culicoides imicola. Insect molecular biology. 2018;27(6):847–61. doi: 10.1111/imb.12526 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

