A Randomized Clinical Trial of Human Papillomavirus Test-and-Treat as Compared to Cytology-Based Screening for Prevention of Cervical Cancer Among Women With Human Immunodeficiency Virus: AIDS Clinical Trials Group Protocol A5282
- PMID: 35294524
- PMCID: PMC9555836
- DOI: 10.1093/cid/ciac213
A Randomized Clinical Trial of Human Papillomavirus Test-and-Treat as Compared to Cytology-Based Screening for Prevention of Cervical Cancer Among Women With Human Immunodeficiency Virus: AIDS Clinical Trials Group Protocol A5282
Abstract
Background: Cytology-based cervical cancer screening followed by confirmation and treatment of biopsy-proven high-grade squamous intraepithelial lesions (bHSIL) is difficult to implement in resource-constrained settings. We hypothesized that high-risk human papillomavirus (hrHPV) testing followed by immediate cryotherapy of women with hrHPV (HPV screen-and-treat) may improve outcomes.
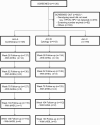
Methods: Randomized, open-label, phase 2, multinational clinical trial enrolling women with human immunodeficiency virus (HIV) age 18 or older with cervical hrHPV and having no cervical lesions or lesions appropriate for cryotherapy. Women were randomized to immediate cryotherapy (Arm A) or cytology-based screening (Arm B). For Arm A, cervical biopsies were obtained followed by cervical cryotherapy, and in Arm B, women with abnormal cytology underwent colposcopy followed by loop electroexcision procedure (LEEP) if bHSIL was detected. Women were followed through 30 months. The primary outcome was time to bHSIL detected from Month 6 through study completion.
Results: In total, 288 women (145 in Arm A, 143 in Arm B) were randomized: median age 35 years, 84% on antiretroviral therapy, median CD4 501 cells/mm3. In Arm A, 39 (27%) of women had bHSIL at entry, and in Arm B, 88 (62%) had abnormal cytology, 22 (15%) were diagnosed with bHSIL, 12 (8%) underwent LEEP. In follow-up, 30 (21%) and 31 (22%) developed bHSIL; time to bHSIL was similar between arms (P=.94). The prevalence of hrHPV at Month 6 was similar between arms (61% and 70%, P=.13).
Conclusions: HPV test-and-treat was not associated with improved bHSIL outcomes as compared to cytology-based screening. More effective treatment options are required to improve outcomes from screen-and-treat programs.
Clinical trials registration: NCT01315363.
Keywords: HIV; HPV; cervical cancer; cytology; women.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. T. J. W. has received grant funding paid to Weill Cornell Medicine from Bristol Myers-Squibb, Gilead Sciences, GlaxoSmithKline/ViiV Healthcare, and Merck Sciences, and has received honoraria from GlaxoSmithKline/ViiV Healthcare and Merck Sciences and reports consulting fees from Merck Sciences. H. C. reports support for attending meetings and/or travel from the NIH paid to the institution. S. G. reports support for the present manuscript from AIDS Clinical Trials Group, DAIDS NIH USA, for trial conduct. R. N. reports support for the present manuscript from AIDS Clinical Trials Group, funded by the Division of AIDS within the NIAID, payments made to the institution to support the ACTG Network’s Coordinating Center at Social & Scientific Systems, Inc. R. W. C. reports grants or contracts UM1 AI068636 and UM1 AI106701 outside of the submitted work. C. F. reports MERCK travel grant for airline tickets/hotel for CROI 2019 to present HPV/Cervical Dysplasia investigator driven study made to airline/hotel. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



References
-
- Parkin DM, Bray F, Ferlay J, Pisani P.. Estimating the world cancer burden: Globocan 2000. Int J Cancer 2001; 94:153–6. - PubMed
-
- Frisch M, Biggar RJ, Goedert JJ.. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and acquired immunodeficiency syndrome. J Natl Cancer Inst 2000; 92:1500–10. - PubMed
-
- Parkin DM, Bray F, Ferlay J, Pisani P.. Global cancer statistics, 2002. CA: Cancer J. Clin. 2005; 55:74–108. - PubMed
-
- Mbulaiteye SM, Katabira ET, Wabinga H, et al. . Spectrum of cancers among HIV-infected persons in Africa: the Uganda AIDS-Cancer Registry Match Study. Int J Cancer 2006; 118:985–90. - PubMed
-
- Sankaranarayanan R, Gaffikin L, Jacob M, Sellors J, Robles S.. A critical assessment of screening methods for cervical neoplasia. Int J Gynaecol Obstet 2005; 89(Suppl 2):S4–S12. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

