A maize triacylglycerol lipase inhibits sugarcane mosaic virus infection
- PMID: 35294544
- PMCID: PMC9157127
- DOI: 10.1093/plphys/kiac126
A maize triacylglycerol lipase inhibits sugarcane mosaic virus infection
Abstract
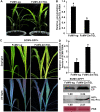
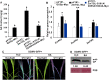
Triacylglycerol lipase (TGL) plays critical roles in providing energy for seed germination and plant development. However, the role of TGL in regulating plant virus infection is largely unknown. In this study, we adopted affinity purification coupled with mass spectrometry and identified that a maize (Zea mays) pathogenesis-related lipase protein Z. mays TGL (ZmTGL) interacted with helper component-proteinase (HC-Pro) of sugarcane mosaic virus (SCMV). Yeast two-hybrid, luciferase complementation imaging, and bimolecular fluorescence complementation assays confirmed that ZmTGL directly interacted with SCMV HC-Pro in vitro and in vivo. The 101-460 residues of SCMV HC-Pro were important for its interaction with ZmTGL. ZmTGL and SCMV HC-Pro co-localized at the mitochondria. Silencing of ZmTGL facilitated SCMV infection, and over-expression of ZmTGL reduced the RNA silencing suppression activity, most likely through reducing HC-Pro accumulation. Our results provided evidence that the lipase hydrolase activity of ZmTGL was associated with reducing HC-Pro accumulation, activation of salicylic acid (SA)-mediated defense response, and inhibition of SCMV infection. We show that ZmTGL inhibits SCMV infection by reducing HC-Pro accumulation and activating the SA pathway.
© American Society of Plant Biologists 2022. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures







References
-
- Abdelkafi S, Barouh N, Fouquet B, Endri F I, Pina M, Scheirlinckx F, Villeneuve P, Carrière F (2011) Carica papaya lipase: a naturally immobilized enzyme with interesting biochemical properties. Plant Foods Hum Nutr 66: 34–40 - PubMed
-
- Baudouin E, Charpenteau M, Roby D, Marco Y, Ranjeva R, Ranty B (1997) Functional expression of a tobacco gene related to the serine hydrolase family – esterase activity towards short-chain dinitrophenyl acylesters. Eur J Biochem 248: 700–706 - PubMed
-
- Blanc S, López-Moya J-J, Wang R, García-Lampasona S, Thornbury DW, Pirone TP (1997) A specific interaction between coat protein and helper component correlates with aphid transmission of a potyvirus. Virology 231: 141–147 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

