Dabigatran versus vitamin K antagonists for atrial fibrillation in clinical practice: final outcomes from Phase III of the GLORIA-AF registry
- PMID: 35294623
- PMCID: PMC9054866
- DOI: 10.1007/s00392-021-01957-1
Dabigatran versus vitamin K antagonists for atrial fibrillation in clinical practice: final outcomes from Phase III of the GLORIA-AF registry
Abstract
Background: Prospectively collected, routine clinical practice-based data on antithrombotic therapy in non-valvular atrial fibrillation (AF) patients are important for assessing real-world comparative outcomes. The objective was to compare the safety and effectiveness of dabigatran versus vitamin K antagonists (VKAs) in patients with newly diagnosed AF.
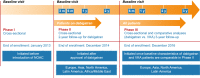
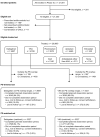
Methods and results: GLORIA-AF is a large, prospective, global registry program. Consecutive patients with newly diagnosed AF and CHA2DS2-VASc scores ≥ 1 were included and followed for 3 years. To control for differences in patient characteristics, the comparative analysis for dabigatran versus VKA was performed on a propensity score (PS)-matched patient set. Missing data were multiply imputed. Proportional-hazards regression was used to estimate hazard ratios (HRs) for outcomes of interest. Between 2014 and 2016, 21,300 eligible patients were included worldwide: 3839 patients were prescribed dabigatran and 4836 VKA with a median age of 71.0 and 72.0 years, respectively; > 85% in each group had a CHA2DS2-VASc-score ≥ 2. The PS-matched comparative analysis for dabigatran and VKA included on average 3326 pairs of matched initiators. For dabigatran versus VKAs, adjusted HRs (95% confidence intervals) were: stroke 0.89 (0.59-1.34), major bleeding 0.61 (0.42-0.88), all-cause death 0.78 (0.63-0.97), and myocardial infarction 0.89 (0.53-1.48). Further analyses stratified by PS and region provided similar results.
Conclusions: Dabigatran was associated with a 39% reduced risk of major bleeding and 22% reduced risk for all-cause death compared with VKA. Stroke and myocardial infarction risks were similar, confirming a more favorable benefit-risk profile for dabigatran compared with VKA in clinical practice. Clinical trial registration https://www.
Clinicaltrials: gov . NCT01468701, NCT01671007.
Keywords: Anticoagulation; Atrial fibrillation; Dabigatran; Stroke prevention; Vitamin K antagonist.
© 2022. The Author(s).
Conflict of interest statement
MVH reports grants from ZonMW Dutch Healthcare Fund, and grants and consultation honoraria from Boehringer Ingelheim, Pfizer/Bristol-Myers Squibb, Bayer Health Care, Aspen, and Daiichi-Sankyo. H-CD reports honoraria for participation in clinical trials, contribution to advisory boards, or oral presentations from Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Medtronic, Pfizer, Portola, and WebMD Global. Financial support for research projects was provided by Boehringer Ingelheim. SJD reports consultancy fees for serving as a Steering Committee member for Boehringer Ingelheim and research grants from Abbott (St Jude Medical). JLH reports consulting fees/honoraria or research support from Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim, Boston Scientific, Daiichi Sankyo Pharma, Janssen Pharmaceuticals, Johnson & Johnson, Medtronic, Pfizer, and Sanofi Aventis. C-SM reports consultancy fees/honoraria from Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Johnson & Johnson, and Pfizer. KJR reports employment by RTI Health Solutions, an independent, non-profit research organization that does work for government agencies and pharmaceutical companies. RL, VKG, and CT report employment by Boehringer Ingelheim International GmbH. DBB reports past employment by Boehringer Ingelheim International GmbH and current employment by UCB pharma. SL reports past employment by Boehringer Ingelheim International GmbH. GYHL reports consultancy for Bayer/Janssen, Bristol-Myers Squibb/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi Sankyo; and speaking for Bayer, Bristol-Myers Squibb/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi Sankyo; no fees are directly received personally.
Figures


References
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

