Pericyte-to-endothelial cell signaling via vitronectin-integrin regulates blood-CNS barrier
- PMID: 35294899
- PMCID: PMC9119930
- DOI: 10.1016/j.neuron.2022.02.017
Pericyte-to-endothelial cell signaling via vitronectin-integrin regulates blood-CNS barrier
Abstract
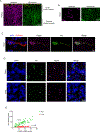
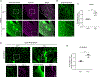
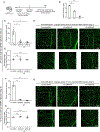
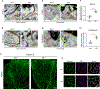
Endothelial cells of blood vessels of the central nervous system (CNS) constitute blood-CNS barriers. Barrier properties are not intrinsic to these cells; rather they are induced and maintained by CNS microenvironment. Notably, the abluminal surfaces of CNS capillaries are ensheathed by pericytes and astrocytes. However, extrinsic factors from these perivascular cells that regulate barrier integrity are largely unknown. Here, we establish vitronectin, an extracellular matrix protein secreted by CNS pericytes, as a regulator of blood-CNS barrier function via interactions with its integrin receptor, α5, in endothelial cells. Genetic ablation of vitronectin or mutating vitronectin to prevent integrin binding, as well as endothelial-specific deletion of integrin α5, causes barrier leakage in mice. Furthermore, vitronectin-integrin α5 signaling maintains barrier integrity by actively inhibiting transcytosis in endothelial cells. These results demonstrate that signaling from perivascular cells to endothelial cells via ligand-receptor interactions is a key mechanism to regulate barrier permeability.
Keywords: barrier; central nervous system; endothelial cells; integrin; pericytes; transcytosis; vitronectin.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
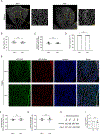
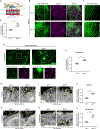
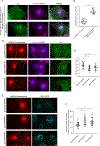
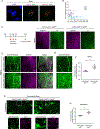
Comment in
-
Matrix proteins plug a hole: How pericytes suppress blood brain barrier transcytosis.Neuron. 2022 May 18;110(10):1601-1603. doi: 10.1016/j.neuron.2022.04.011. Neuron. 2022. PMID: 35588710 Free PMC article.
References
-
- Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. (2010). Pericytes regulate the blood-brain barrier. Nature 468, 557–561. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

