Non-rolling flag leaves use an effective mechanism to reduce water loss and light-induced damage under drought stress
- PMID: 35294964
- PMCID: PMC9486892
- DOI: 10.1093/aob/mcac035
Non-rolling flag leaves use an effective mechanism to reduce water loss and light-induced damage under drought stress
Abstract
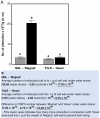
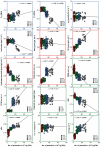
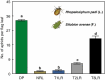
Background and aims: The study reports on four different types of flag leaf rolling under soil drought in relation to the level of cell wall-bound phenolics. The flag leaf colonization by aphids, as a possible bioindicator of the accumulation of cell wall-bound phenolics, was also estimated.
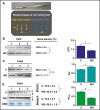
Methods: The proteins of the photosynthetic apparatus that form its core and are crucial for maintaining its stability (D1/PsbA protein), limit destructive effects of light (PsbS, a protein binding carotenoids in the antennas) and participate in efficient electron transport between photosystems II (PSII) and PSI (Rieske iron-sulfur protein of the cytochrome b6f complex) were evaluated in two types of flag leaf rolling. Additionally, biochemical and physiological reactions to drought stress in rolling and non-rolling flag leaves were compared.
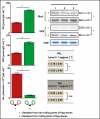
Key results: The study identified four types of genome-related types of flag leaf rolling. The biochemical basis for these differences was a different number of phenolic molecules incorporated into polycarbohydrate structures of the cell wall. In an extreme case of non-rolling dehydrated flag leaves, they were found to accumulate high amounts of cell wall-bound phenolics that limited cell water loss and protected the photosynthetic apparatus against excessive light. PSII was also additionally protected against excess light by the accumulation of photosynthetic apparatus proteins that ensured stable and efficient transport of excitation energy beyond PSII and its dissipation as far-red fluorescence and heat. Our analysis revealed a new type of flag leaf rolling brought about by an interaction between wheat and rye genomes, and resulting in biochemical specialization of flexible, rolling and rigid, non-rolling parts of the flag leaf. The study confirmed limited aphid colonization of the flag leaves with enhanced content of cell wall-bound phenolics.
Conclusions: Non-rolling leaves developed effective adaptation mechanisms to reduce both water loss and photoinhibitory damage to the photosynthetic apparatus under drought stress.
Keywords: Blue and red fluorescence; cell wall phenolics; chlorophyll fluorescence; flag leaf rolling; hydrogen peroxide; photosynthetic apparatus proteins; triticale.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Annals of Botany Company. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures






References
-
- Agati G, Cerovic ZG, Moya I. 2000. The effect of decreasing temperature up to chilling values on the in vivo F685/F735 chlorophyll fluorescence ratio in Phaseolus vulgaris an Pisum sativum: the role of the photosystem I contribution to the 735 nm fluorescence band. Photochemistry and Photobiology 72: 75–84. - PubMed
-
- Arnold T, Appel H, Patel V, Stocum E, Kavalier A, Schultz J. 2004. Carbohydrate translocation determines the phenolic content of Populus foliage: a test of the sink–source model of plant defense. New Phytologist 164: 157–164. - PubMed
-
- Ashwell G. 1957. Colorimetric analysis of sugars. Methods in Enzymology 3: 73–105.
-
- Baker NR. 1991. A possible role for photosystem II in environmental perturbations of photosynthesis. Physiologia Plantarum 81: 563–570.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

