Multivitamins in the prevention of cancer and cardiovascular disease: the COcoa Supplement and Multivitamin Outcomes Study (COSMOS) randomized clinical trial
- PMID: 35294969
- PMCID: PMC9170475
- DOI: 10.1093/ajcn/nqac056
Multivitamins in the prevention of cancer and cardiovascular disease: the COcoa Supplement and Multivitamin Outcomes Study (COSMOS) randomized clinical trial
Abstract
Background: Although older adults commonly take multivitamin-multimineral (MVM) supplements to promote health, evidence on the use of daily MVMs on invasive cancer is limited.
Objectives: The study objective was to determine if a daily MVM decreases total invasive cancer among older adults.
Methods: We performed a randomized, double-blind, placebo-controlled, 2-by-2 factorial trial of a daily MVM and cocoa extract for prevention of cancer and cardiovascular disease (CVD) among 21,442 US adults (12,666 women aged ≥65 y and 8776 men aged ≥60 y) free of major CVD and recently diagnosed cancer. The intervention phase was from June 2015 through December 2020. This article reports on the MVM intervention. Participants were randomly assigned to daily MVM or placebo. The primary outcome was total invasive cancer, excluding nonmelanoma skin cancer. Secondary outcomes included major site-specific cancers, total CVD, all-cause mortality, and total cancer risk among those with a baseline history of cancer.
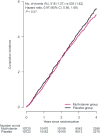
Results: During a median follow-up of 3.6 y, invasive cancer occurred in 518 participants in the MVM group and 535 participants in the placebo group (HR: 0.97; 95% CI: 0.86, 1.09; P = 0.57). We observed no significant effect of a daily MVM on breast cancer (HR: 1.06; 95% CI: 0.79, 1.42) or colorectal cancer (HR: 1.30; 95% CI: 0.80, 2.12). We observed a protective effect of a daily MVM on lung cancer (HR: 0.62; 95% CI: 0.42, 0.92). The composite CVD outcome occurred in 429 participants in the MVM group and 437 participants in the placebo group (HR: 0.98; 95% CI: 0.86, 1.12). MVM use did not significantly affect all-cause mortality (HR: 0.93; 95% CI: 0.81, 1.08). There were no safety concerns.
Conclusions: A daily MVM supplement, compared with placebo, did not significantly reduce the incidence of total cancer among older men and women. Future studies are needed to determine the effects of MVMs on other aging-related outcomes among older adults. This trial is registered at www.clinicaltrials.gov as NCT02422745.
Keywords: cancer; cardiovascular disease; cocoa extract; flavanols; multivitamin; randomized clinical trial.
© The Author(s) 2022. Published by Oxford University Press on behalf of the American Society for Nutrition.
Figures


References
-
- Yetley EA. Multivitamin and multimineral dietary supplements: definitions, characterization, bioavailability, and drug interactions. Am J Clin Nutr. 2007;85(1):269S–76S. - PubMed
-
- Gahche J, Bailey R, Burt V, Hughes J, Yetley E, Dwyer J, Picciano MF, McDowell M, Sempos C. Dietary supplement use among U.S. adults has increased since NHANES III (1988–1994). NCHS Data Brief. 2011;61:1–8. - PubMed
-
- Rautiainen S, Manson JE, Lichtenstein AH, Sesso HD. Dietary supplements and disease prevention—a global overview. Nat Rev Endocrinol. 2016;12(7):407–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- 75N92021D00001/HL/NHLBI NIH HHS/United States
- 75N92020D00005/HL/NHLBI NIH HHS/United States
- 75N92021D00005/WH/WHI NIH HHS/United States
- 75N98021D00005/OD/NIH HHS/United States
- 75N92021D00003/WH/WHI NIH HHS/United States
- R01 HL157665/HL/NHLBI NIH HHS/United States
- 75N92022D00005/HL/NHLBI NIH HHS/United States
- K24 HL136852/HL/NHLBI NIH HHS/United States
- R01 EY025623/EY/NEI NIH HHS/United States
- 75N92021D00002/HL/NHLBI NIH HHS/United States
- 75N92021D00004/WH/WHI NIH HHS/United States
- R01 AG071611/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

