Rapamycin Improved Retinal Function and Morphology in a Mouse Model of Retinal Degeneration
- PMID: 35295093
- PMCID: PMC8919089
- DOI: 10.3389/fnins.2022.846584
Rapamycin Improved Retinal Function and Morphology in a Mouse Model of Retinal Degeneration
Abstract
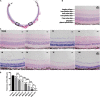
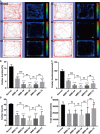
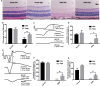
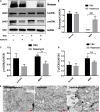
The retina is an important visual organ, which is responsible for receiving light signals and transmitting them to the optic nerve center step by step. The retina contains a variety of cells, among which photoreceptor cells receive light signals and convert them into nerve signals, and are mainly responsible for light and dark vision. Retinal degeneration is mainly the degeneration of photoreceptor cells, and retinitis pigmentosa (RP) is characterized by rod degeneration followed by cone degeneration. So far, there is still a lack of effective drugs to treat RP. Here, we established a stable RP model by tail vein injection of methyl methanesulfonate to study the mechanism of retinal photoreceptor degeneration. Mechanistic target of rapamycin (mTOR) is located in the central pathway of growth and energy metabolism and changes in a variety of diseases in response to pathological changes. We found that the mTOR was activated in this model. Therefore, the inhibitor of mTOR, rapamycin was used to suppress the expression of mTOR and interfere with photoreceptor degeneration. Electroretinogram assay showed that the function of mice retina was improved. Hematoxylin and eosin staining results displayed that retinal photoreceptor thickness and morphology were improved. Also, the autophagy in rapamycin group was activated, which revealed that rapamycin may protect the retinal photoreceptor by inhibiting mTOR and then activating autophagy.
Keywords: MTOR; autophagy; photoreceptor; rapamycin; retinal degeneration.
Copyright © 2022 Zhao, Lv, Yang and Peng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Mice deficient in UXT exhibit retinitis pigmentosa-like features via aberrant autophagy activation.Autophagy. 2021 Aug;17(8):1873-1888. doi: 10.1080/15548627.2020.1796015. Epub 2020 Aug 2. Autophagy. 2021. PMID: 32744119 Free PMC article.
-
Inhibition of mTOR signaling by rapamycin protects photoreceptors from degeneration in rd1 mice.Zool Res. 2021 Jul 18;42(4):482-486. doi: 10.24272/j.issn.2095-8137.2021.049. Zool Res. 2021. PMID: 34235896 Free PMC article.
-
Wheel running exercise protects against retinal degeneration in the I307N rhodopsin mouse model of inducible autosomal dominant retinitis pigmentosa.Mol Vis. 2019 Aug 21;25:462-476. eCollection 2019. Mol Vis. 2019. PMID: 31523123 Free PMC article.
-
[New drug therapy for retinal degeneration].Nippon Ganka Gakkai Zasshi. 2008 Jan;112(1):7-21. Nippon Ganka Gakkai Zasshi. 2008. PMID: 18240599 Review. Japanese.
-
Defective cone photoreceptor cytoskeleton, alignment, feedback, and energetics can lead to energy depletion in macular degeneration.Prog Retin Eye Res. 2004 Sep;23(5):495-522. doi: 10.1016/j.preteyeres.2004.04.005. Prog Retin Eye Res. 2004. PMID: 15302348 Review.
Cited by
-
Pten Loss Triggers Progressive Photoreceptor Degeneration in an mTORC1-Independent Manner.Invest Ophthalmol Vis Sci. 2025 Mar 3;66(3):45. doi: 10.1167/iovs.66.3.45. Invest Ophthalmol Vis Sci. 2025. PMID: 40116678 Free PMC article.
-
Beyond Genetics: The Role of Metabolism in Photoreceptor Survival, Development and Repair.Front Cell Dev Biol. 2022 May 18;10:887764. doi: 10.3389/fcell.2022.887764. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35663397 Free PMC article. Review.
-
AUTOPHAGY IN THE EYE: FROM PHYSIOLOGY TO PATHOPHYSOLOGY.Autophagy Rep. 2023;2(1):2178996. doi: 10.1080/27694127.2023.2178996. Epub 2023 Mar 1. Autophagy Rep. 2023. PMID: 37034386 Free PMC article.
References
-
- Brown E. J., Beal P. A., Keith C. T., Chen J., Shin T. B., Schreiber S. L. (1995). Control of p70 s6 kinase by kinase activity of FRAP in vivo. Nature 377 441–446. - PubMed
-
- Chang B., Hawes N. L., Hurd R. E., Davisson M. T., Nusinowitz S., Heckenlively J. R. (2002). Retinal degeneration mutants in the mouse. Vis. Res. 42 517–525. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

