An Adverse Outcome Pathway for Decreased Lung Function Focusing on Mechanisms of Impaired Mucociliary Clearance Following Inhalation Exposure
- PMID: 35295103
- PMCID: PMC8915806
- DOI: 10.3389/ftox.2021.750254
An Adverse Outcome Pathway for Decreased Lung Function Focusing on Mechanisms of Impaired Mucociliary Clearance Following Inhalation Exposure
Abstract
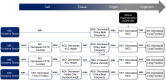
Adverse outcome pathways (AOPs) help to organize available mechanistic information related to an adverse outcome into key events (KEs) spanning all organizational levels of a biological system(s). AOPs, therefore, aid in the biological understanding of a particular pathogenesis and also help with linking exposures to eventual toxic effects. In the regulatory context, knowledge of disease mechanisms can help design testing strategies using in vitro methods that can measure or predict KEs relevant to the biological effect of interest. The AOP described here evaluates the major processes known to be involved in regulating efficient mucociliary clearance (MCC) following exposures causing oxidative stress. MCC is a key aspect of the innate immune defense against airborne pathogens and inhaled chemicals and is governed by the concerted action of its functional components, the cilia and airway surface liquid (ASL). The AOP network described here consists of sequences of KEs that culminate in the modulation of ciliary beat frequency and ASL height as well as mucus viscosity and hence, impairment of MCC, which in turn leads to decreased lung function.
Keywords: AOP; NAMs; adverse outcome pathway; ciliary beat frequency; inhalation toxicity; lung function; mucociliary clearance; new approach methodologies.
Copyright © 2021 Luettich, Sharma, Yepiskoposyan, Breheny and Lowe.
Conflict of interest statement
KL and HY are employed by Philip Morris International (PMI). MS is employed by PETA International Science Consortium Ltd. DB is employed by British American Tobacco (Investments) Ltd. FJL is employed by Broughton Nicotine Services LLC.
Figures
Similar articles
-
Role of cilia, mucus, and airway surface liquid in mucociliary dysfunction: lessons from mouse models.J Aerosol Med Pulm Drug Deliv. 2008 Mar;21(1):13-24. doi: 10.1089/jamp.2007.0659. J Aerosol Med Pulm Drug Deliv. 2008. PMID: 18518828 Review.
-
Effects of second hand smoke on airway secretion and mucociliary clearance.Front Physiol. 2012 Aug 28;3:342. doi: 10.3389/fphys.2012.00342. eCollection 2012. Front Physiol. 2012. PMID: 22973232 Free PMC article.
-
Muco-ciliary clearance: A review of modelling techniques.J Biomech. 2020 Jan 23;99:109578. doi: 10.1016/j.jbiomech.2019.109578. Epub 2019 Dec 24. J Biomech. 2020. PMID: 31916998 Review.
-
Linking increased airway hydration, ciliary beating, and mucociliary clearance through ENaC inhibition.Am J Physiol Lung Cell Mol Physiol. 2015 Jan 1;308(1):L22-32. doi: 10.1152/ajplung.00163.2014. Epub 2014 Oct 31. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25361567 Free PMC article.
-
Continuous mucociliary transport by primary human airway epithelial cells in vitro.Am J Physiol Lung Cell Mol Physiol. 2015 Jul 15;309(2):L99-108. doi: 10.1152/ajplung.00024.2015. Epub 2015 May 15. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25979076 Free PMC article.
Cited by
-
New approach methodologies (NAMs) for the in vitro assessment of cleaning products for respiratory irritation: workshop report.Front Toxicol. 2024 Oct 8;6:1431790. doi: 10.3389/ftox.2024.1431790. eCollection 2024. Front Toxicol. 2024. PMID: 39439531 Free PMC article. Review.
-
Evaluation of behavioural, chemical, toxicological and clinical studies of a tobacco heated product glo™ and the potential for bridging from a foundational dataset to new product iterations.Toxicol Rep. 2022 Jun 25;9:1426-1442. doi: 10.1016/j.toxrep.2022.06.014. eCollection 2022. Toxicol Rep. 2022. PMID: 36561950 Free PMC article. Review.
-
Differences in the anatomy and physiology of the human and rat respiratory tracts and impact on toxicological assessments.Regul Toxicol Pharmacol. 2024 Jun;150:105648. doi: 10.1016/j.yrtph.2024.105648. Epub 2024 May 20. Regul Toxicol Pharmacol. 2024. PMID: 38772524 Free PMC article. Review.
-
Use of quantitative in vitro to in vivo extrapolation (QIVIVE) for the assessment of non-combustible next-generation product aerosols.Front Toxicol. 2024 Apr 11;6:1373325. doi: 10.3389/ftox.2024.1373325. eCollection 2024. Front Toxicol. 2024. PMID: 38665213 Free PMC article.
-
Use of new approach methodologies (NAMs) to meet regulatory requirements for the assessment of tobacco and other nicotine-containing products.Front Toxicol. 2022 Sep 9;4:943358. doi: 10.3389/ftox.2022.943358. eCollection 2022. Front Toxicol. 2022. PMID: 36157974 Free PMC article.
References
LinkOut - more resources
Full Text Sources

