Effects of Delta-9 Tetrahydrocannabinol (THC) on Oocyte Competence and Early Embryonic Development
- PMID: 35295104
- PMCID: PMC8915882
- DOI: 10.3389/ftox.2021.647918
Effects of Delta-9 Tetrahydrocannabinol (THC) on Oocyte Competence and Early Embryonic Development
Abstract
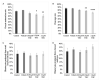
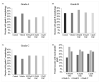
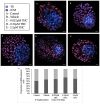
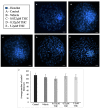
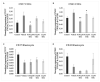
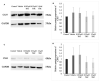
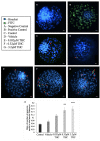
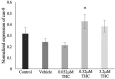
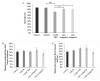
Recent changes in legal status and public perception of cannabis have contributed to an increase use amongst women of reproductive age. Concurrently, there is inadequate evidence-based knowledge to guide clinical practice regarding cannabis and its effects on fertility and early embryonic development. This study aimed to evaluate the effects of the primary psychoactive component of cannabis, delta-9 tetrahydrocannabinol (THC), during oocyte maturation, and its impact on the developing embryo. Bovine oocytes were matured in vitro for 24 h under clinically relevant doses of THC mimicking plasma levels achieved after therapeutic (0.032 μM) and recreational (0.32 and 3.2 μM) cannabis use. THC-treated oocytes were assessed for development and quality parameters at both the oocyte and embryo level. Characteristics of oocytes treated with cannabinoid receptor antagonists were also assessed. Oocytes treated with 0.32 and 3.2 μM THC, were significantly less likely to reach metaphase II (p < 0.01) and consequently had lower cleavage rates at day 2 post-fertilization (p < 0.0001). Treatment with cannabinoid receptor antagonists restored this effect (p < 0.05). Oocytes that did reach MII showed no differences in spindle morphology. Oocytes treated with 0.032 μM THC had significantly lower connexin mRNA (p < 0.05) (correlated with decreased quality), but this was not confirmed at the protein level. At the blastocyst stage there were no significant differences in developmental rates or the proportion of trophectoderm to inner cell mass cells between the control and treatment groups. These blastocysts, however, displayed an increased level of apoptosis in the 0.32 and 3.2 μM groups (p < 0.0001). Our findings suggest a possible disruptive effect of cannabis on oocyte maturation and early embryonic development.
Keywords: THC; cannabis; embryo; fertility; oocyte.
Copyright © 2021 Misner, Taborek, Dufour, Sharifi, Khokhar and Favetta.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
THC and sperm: Impact on fertilization capability, pre-implantation in vitro development and epigenetic modifications.PLoS One. 2024 Mar 27;19(3):e0298697. doi: 10.1371/journal.pone.0298697. eCollection 2024. PLoS One. 2024. PMID: 38536780 Free PMC article.
-
Mitochondria-targeted therapy rescues development and quality of embryos derived from oocytes matured under oxidative stress conditions: a bovine in vitro model.Hum Reprod. 2019 Oct 2;34(10):1984-1998. doi: 10.1093/humrep/dez161. Hum Reprod. 2019. PMID: 31625574
-
Tetrahydrocannabinol Modulates in Vitro Maturation of Oocytes and Improves the Blastocyst Rates after in Vitro Fertilization.Cell Physiol Biochem. 2019;53(3):439-452. doi: 10.33594/000000149. Cell Physiol Biochem. 2019. PMID: 31436397
-
Effects of in vivo prematuration and in vivo final maturation on developmental capacity and quality of pre-implantation embryos.Theriogenology. 2002 Jan 1;57(1):5-20. doi: 10.1016/s0093-691x(01)00655-0. Theriogenology. 2002. PMID: 11775980 Review.
-
Glucocorticoids, Stress and Delta-9 Tetrahydrocannabinol (THC) during Early Embryonic Development.Int J Mol Sci. 2021 Jul 7;22(14):7289. doi: 10.3390/ijms22147289. Int J Mol Sci. 2021. PMID: 34298908 Free PMC article. Review.
Cited by
-
The effects of cannabis and cannabinoids on the endocrine system.Rev Endocr Metab Disord. 2022 Jun;23(3):401-420. doi: 10.1007/s11154-021-09682-w. Epub 2021 Aug 30. Rev Endocr Metab Disord. 2022. PMID: 34460075 Review.
-
Cannabis significantly alters DNA methylation of the human ovarian follicle in a concentration-dependent manner.Mol Hum Reprod. 2022 Jun 30;28(7):gaac022. doi: 10.1093/molehr/gaac022. Mol Hum Reprod. 2022. PMID: 35674367 Free PMC article.
-
The Silent Threat to Women's Fertility: Uncovering the Devastating Effects of Oxidative Stress.Antioxidants (Basel). 2023 Jul 26;12(8):1490. doi: 10.3390/antiox12081490. Antioxidants (Basel). 2023. PMID: 37627485 Free PMC article. Review.
-
DNA methylation, but not microRNA expression, is affected by in vitro THC exposure in bovine granulosa cells.BMC Pharmacol Toxicol. 2024 Jul 15;25(1):42. doi: 10.1186/s40360-024-00763-5. BMC Pharmacol Toxicol. 2024. PMID: 39010179 Free PMC article.
-
A narrative review of the ethnomedicinal usage of Cannabis sativa Linnaeus as traditional phytomedicine by folk medicine practitioners of Bangladesh.J Cannabis Res. 2021 Mar 19;3(1):8. doi: 10.1186/s42238-021-00063-3. J Cannabis Res. 2021. PMID: 33741060 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

