Micro and Nanoplastics Identification: Classic Methods and Innovative Detection Techniques
- PMID: 35295124
- PMCID: PMC8915801
- DOI: 10.3389/ftox.2021.636640
Micro and Nanoplastics Identification: Classic Methods and Innovative Detection Techniques
Abstract
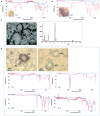
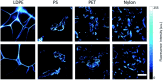
Micro and nanoplastics are fragments with dimensions less than a millimeter invading all terrestrial and marine environments. They have become a major global environmental issue in recent decades and, indeed, recent scientific studies have highlighted the presence of these fragments all over the world even in environments that were thought to be unspoiled. Analysis of micro/nanoplastics in isolated samples from abiotic and biotic environmental matrices has become increasingly common. Hence, the need to find valid techniques to identify these micro and nano-sized particles. In this review, we discuss the current and potential identification methods used in microplastic analyses along with their advantages and limitations. We discuss the most suitable techniques currently available, from physical to chemical ones, as well as the challenges to enhance the existing methods and develop new ones. Microscopical techniques (i.e., dissect, polarized, fluorescence, scanning electron, and atomic force microscopy) are one of the most used identification methods for micro/nanoplastics, but they have the limitation to produce incomplete results in analyses of small particles. At present, the combination with chemical analysis (i.e., spectroscopy) overcome this limit together with recently introduced alternative approaches. For example, holographic imaging in microscope configuration images microplastics directly in unfiltered water, thus discriminating microplastics from diatoms and differentiates different sizes, shapes, and plastic types. The development of new analytical instruments coupled with each other or with conventional and innovative microscopy could solve the current problems in the identification of micro/nanoplastics.
Keywords: analytical methods; characterization; environmental matrices; microplastics; microscopy; nanoplastics; spectroscopy.
Copyright © 2021 Mariano, Tacconi, Fidaleo, Rossi and Dini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Advances in Ultra-Trace Analytical Capability for Micro/Nanoplastics and Water-Soluble Polymers in the Environment: Fresh Falling Urban Snow.Environ Pollut. 2021 May 1;276:116698. doi: 10.1016/j.envpol.2021.116698. Epub 2021 Feb 9. Environ Pollut. 2021. PMID: 33611197
-
A review on constructive classification framework of research trends in analytical instrumentation for secondary micro(nano)plastics: What is new and what needs next?Environ Pollut. 2023 Oct 15;335:122320. doi: 10.1016/j.envpol.2023.122320. Epub 2023 Aug 4. Environ Pollut. 2023. PMID: 37544402 Review.
-
The plastic brain: neurotoxicity of micro- and nanoplastics.Part Fibre Toxicol. 2020 Jun 8;17(1):24. doi: 10.1186/s12989-020-00358-y. Part Fibre Toxicol. 2020. PMID: 32513186 Free PMC article. Review.
-
Raman Tweezers for Small Microplastics and Nanoplastics Identification in Seawater.Environ Sci Technol. 2019 Aug 6;53(15):9003-9013. doi: 10.1021/acs.est.9b03105. Epub 2019 Jul 12. Environ Sci Technol. 2019. PMID: 31259538
-
Chemical Analysis of Microplastics and Nanoplastics: Challenges, Advanced Methods, and Perspectives.Chem Rev. 2021 Oct 13;121(19):11886-11936. doi: 10.1021/acs.chemrev.1c00178. Epub 2021 Aug 26. Chem Rev. 2021. PMID: 34436873 Review.
Cited by
-
High-throughput microplastic assessment using polarization holographic imaging.Sci Rep. 2024 Jan 29;14(1):2355. doi: 10.1038/s41598-024-52762-5. Sci Rep. 2024. PMID: 38287056 Free PMC article.
-
Control of Nanoparticle Size of Intrinsically Fluorescent PET (Polyethylene Terephthalate) Particles Produced Through Nanoprecipitation.Molecules. 2025 Jan 13;30(2):282. doi: 10.3390/molecules30020282. Molecules. 2025. PMID: 39860152 Free PMC article.
-
Addressing the relevance of polystyrene nano- and microplastic particles used to support exposure, toxicity and risk assessment: implications and recommendations.Part Fibre Toxicol. 2024 Sep 27;21(1):39. doi: 10.1186/s12989-024-00599-1. Part Fibre Toxicol. 2024. PMID: 39334292 Free PMC article. Review.
-
Micro- and Nanoplastics Produced from Textile Finishes: A Review.Langmuir. 2024 Aug 16;40(34):17849-67. doi: 10.1021/acs.langmuir.4c00552. Online ahead of print. Langmuir. 2024. PMID: 39151927 Free PMC article. Review.
-
Fluorescent Nanodiamonds for Tracking Single Polymer Particles in Cells and Tissues.Anal Chem. 2023 Sep 5;95(35):13046-13054. doi: 10.1021/acs.analchem.3c01452. Epub 2023 Aug 23. Anal Chem. 2023. PMID: 37612789 Free PMC article.
References
-
- Bianco V., Memmolo P., Carcagn I. P., Merola F., Paturzo M., Distante C., et al. . (2020). Microplastic identification via holographic imaging and machine learning. Adv. Intell. Syst. 2:1900153. 10.1002/aisy.201900153 - DOI
Publication types
LinkOut - more resources
Full Text Sources

