The Nervous System Contributes to the Tumorigenesis and Progression of Human Digestive Tract Cancer
- PMID: 35295188
- PMCID: PMC8920690
- DOI: 10.1155/2022/9595704
The Nervous System Contributes to the Tumorigenesis and Progression of Human Digestive Tract Cancer
Abstract
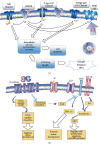
Tumors of the gastrointestinal tract are one of the highest incidences of morbidity and mortality in humans. Recently, a growing number of researchers have indicated that nerve fibers and nerve signals participate in tumorigenesis. The current overarching view based on the responses to therapy revealed that tumors are partly promoted by the tumor microenvironment (TME), endogenous oncogenic factors, and complex systemic processes. Homeostasis of the neuroendocrine-immune axis (NEI axis) maintains a healthy in vivo environment in humans, and dysfunction of the axis contributes to various cancers, including the digestive tract. Interestingly, nerves might promote tumor development via multiple mechanisms, including perineural invasion (PNI), central level regulation, NEI axis effect, and neurotransmitter induction. This review focuses on the association between digestive tumors and nerve regulation, including PNI, the NEI axis, stress, and neurotransmitters, as well as on the potential clinical application of neurotherapy, aiming to provide a new perspective on the management of digestive cancers.
Copyright © 2022 Dayou Dai and Hao Liu.
Conflict of interest statement
We declare that we have no conflict of interest.
Figures
Similar articles
-
Nerve-tumor crosstalk in tumor microenvironment: From tumor initiation and progression to clinical implications.Biochim Biophys Acta Rev Cancer. 2024 Jul;1879(4):189121. doi: 10.1016/j.bbcan.2024.189121. Epub 2024 May 23. Biochim Biophys Acta Rev Cancer. 2024. PMID: 38796026 Review.
-
Unveiling the pathogenesis of perineural invasion from the perspective of neuroactive molecules.Biochem Pharmacol. 2021 Jun;188:114547. doi: 10.1016/j.bcp.2021.114547. Epub 2021 Apr 8. Biochem Pharmacol. 2021. PMID: 33838132 Review.
-
Vagus innervation in the gastrointestinal tumor: Current understanding and challenges.Biochim Biophys Acta Rev Cancer. 2023 May;1878(3):188884. doi: 10.1016/j.bbcan.2023.188884. Epub 2023 Mar 28. Biochim Biophys Acta Rev Cancer. 2023. PMID: 36990250 Review.
-
The association between the neuroendocrine system and the tumor immune microenvironment: Emerging directions for cancer immunotherapy.Biochim Biophys Acta Rev Cancer. 2023 Nov;1878(6):189007. doi: 10.1016/j.bbcan.2023.189007. Epub 2023 Oct 30. Biochim Biophys Acta Rev Cancer. 2023. PMID: 37907132 Review.
-
The nerve cells in gastrointestinal cancers: from molecular mechanisms to clinical intervention.Oncogene. 2024 Jan;43(2):77-91. doi: 10.1038/s41388-023-02909-x. Epub 2023 Dec 11. Oncogene. 2024. PMID: 38081962 Free PMC article. Review.
Cited by
-
Crosstalk Between the Nervous System and Colorectal Cancer.Neurosci Bull. 2025 Jan;41(1):93-106. doi: 10.1007/s12264-024-01238-7. Epub 2024 Jun 16. Neurosci Bull. 2025. PMID: 38879846 Review.
-
Dopamine receptors gene overexpression in the microenvironment of invasive gastric cancer and its potential implications.Mol Biol Rep. 2023 Aug;50(8):6529-6542. doi: 10.1007/s11033-023-08541-y. Epub 2023 Jun 18. Mol Biol Rep. 2023. PMID: 37330941
-
Nestin Expression and the Survival of Patients With Digestive Tract Cancers: A Systematic Review and Meta-Analysis.Turk J Gastroenterol. 2023 Sep;34(9):902-910. doi: 10.5152/tjg.2023.22485. Turk J Gastroenterol. 2023. PMID: 37485559 Free PMC article.
-
Enhancing therapeutic efficacy in triple-negative breast cancer and melanoma: synergistic effects of modulated electro-hyperthermia (mEHT) with NSAIDs especially COX-2 inhibition in in vivo models.Mol Oncol. 2024 Apr;18(4):1012-1030. doi: 10.1002/1878-0261.13585. Epub 2024 Jan 12. Mol Oncol. 2024. PMID: 38217262 Free PMC article.
References
-
- Saloman J. L., Albers K. M., Li D., et al. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proceedings of the National Academy of Sciences of the United States of America . 2016;113(11):3078–3083. doi: 10.1073/pnas.1512603113. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

