Open-Label Pilot Study of a Single Intra-Articular Injection of Mannitol-Modified Cross-Linked Hyaluronic Acid (HANOX-M-XL) for the Treatment of the First Metatarsophalangeal Osteoarthritis (Hallux Rigidus): The REPAR Trial
- PMID: 35295206
- PMCID: PMC8918964
- DOI: 10.1177/11795441211055882
Open-Label Pilot Study of a Single Intra-Articular Injection of Mannitol-Modified Cross-Linked Hyaluronic Acid (HANOX-M-XL) for the Treatment of the First Metatarsophalangeal Osteoarthritis (Hallux Rigidus): The REPAR Trial
Abstract
Purpose: The purpose of this study was to obtain information on safety and short-term efficiency of a single intra-articular injection of mannitol-modified cross-linked hyaluronic acid (HANOX-M-XL) in patients with painful first metatarsophalangeal joint osteoarthritis (1stMTPJ-OA).
Methods: The study involved an observational, single-arm, prospective multicentre trial, with a 3-month follow-up. Inclusion criteria were patients with symptomatic 1st MTPJ-OA not relieved by analgesics and / or non-steroidal-anti-inflammatory drugs and / or foot orthotic. All patients received a single, imaging-guided intra-articular (IA) injection of 1 mL of HANOX-M-XL in the 1st MTPJ. The primary outcome was the change in pain between the date of injection and month 3. The secondary outcomes were the patient assessment of effectiveness, the decrease in painkiller use and the influence of the radiographic score on the clinical efficacy.
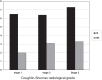
Results: Sixty-five participants (72.3% women, mean age = 60) were included in the trial. Coughlin-Shurnas radiological grade was 1 in 28 patients, 2 in 29, and 3 in 6. At baseline and month 3, the average pain (0-10) was 6.5 ± 1.8 and 2.8 ± 2.3, respectively. The change in pain score was highly significant (-3.1 ± 2.9; P < .0001). At baseline there was no statistically difference in pain between the radiological stages (P = .69). At endpoint, the average pain score was 2.0 ± 1.9 in x-ray stage 1, 3.1 ± 2.3 in stage 2 and 3.3 ± 2.4 in stage 3 (P = .001). Mild to moderate adverse reactions were reported by 15 patients. All were a transient increase of the hallux pain that occurred immediately and up to 6 hours after injection and resolved in 1 to 7 days.
Conclusion: This pilot study suggests that a single IA injection of HANOX-M-XL is safe and mainly benefits patients with mild moderate 1st MTPJ-OA. Further randomized controlled trials are necessary to confirm these preliminary encouraging results.
Keywords: Hyaluronic acid; foot; hallux rigidus; injections; intra-articular; metatarsophalangeal joint; osteoarthritis; viscosupplementation.
© The Author(s) 2022.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Laurent Galois did not receive remuneration for his contribution to the article. Jean-Yves Coillard did not receive remuneration for his contribution to the article. Jérôme Porterie did not receive remuneration for his contribution to the article. Sylvie Melac-Ducamp did not receive remuneration for her contribution to the article. Thierry Conrozier received fees from LABRHA for scientific, speaker and board member services.
Figures



References
-
- Davies-Colley N. Contraction of the metatarsophalangeal joint of the great toe. Br Med J. 1887;1:728.
-
- Coughlin MJ, Shurnas PS. Hallux rigidus: demographics, etiology, and radiographic assessment. Foot Ankle Int. 2003;24:731-743. - PubMed
-
- Weinfeld SB, Schon LC. Hallux metatarsophalangeal arthritis. Clin Orthop Relat Res. 1998;349:9-19. - PubMed
LinkOut - more resources
Full Text Sources

