Assessing cough symptom severity in refractory or unexplained chronic cough: findings from patient focus groups and an international expert panel
- PMID: 35295233
- PMCID: PMC8918938
- DOI: 10.1183/23120541.00667-2021
Assessing cough symptom severity in refractory or unexplained chronic cough: findings from patient focus groups and an international expert panel
Abstract
Background: Cough symptom severity represents an important subjective end-point to assess the impact of therapies for patients with refractory or unexplained chronic cough (RCC/UCC). As existing instruments assessing the severity of cough are neither widely available nor tested for measurement properties, we aim to develop a new patient-reported outcome measure addressing cough severity.
Objective: The aim of this study was to establish items and domains that would inform development of a new cough severity instrument.
Methods: Three focus groups involving 16 adult patients with RCC/UCC provided data that we analysed using directed content analysis. Discussions led to consensus among an international panel of 15 experts on candidate items and domains to assess cough severity.
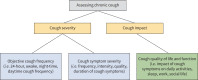
Results: The patient focus group provided 48 unique items arranged under broad domains of urge-to-cough sensations and cough symptom. Feedback from expert panel members confirmed the appropriateness of items and domains, and provided an additional subdomain related to cough triggers. The final conceptual framework comprised 51 items in the following domains: urge-to-cough sensations (subdomains: frequency and intensity) and cough symptom (subdomains: triggers, control, frequency, fit/bout duration, intensity, quality and associated features/sequelae).
Conclusions: Consensus findings from patients and international experts established domains of urge-to-cough and cough symptom with associated subdomains and relevant items. The results support item generation and content validity for a novel patient-reported outcome measure for use in health research and clinical practice.
Copyright ©The authors 2022.
Conflict of interest statement
Conflicts of interest: E. Kum, G.H. Guyatt, C. Munoz, S. Beaudin, S-A. Li, R. Abdulqawi, H. Badri, L. Dupont and L. McGarvey report no conflicts of interest. L-P. Boulet reports grants from Amgen, AstraZeneca, GlaxoSmithKline, Merck, Novartis and Sanofi-Regeneron, and personal fees from AstraZeneca, Covis, Novartis, GlaxoSmithKline, Merck and Sanofi-Regeneron, outside the submitted work. R. Chen reports grants and personal fees from AstraZeneca and GlaxoSmithKline, and personal fees from Novartis and Merck, outside the submitted work. P. Dicpinigaitis reports consulting fees from Merck, Bellus, Bayer, Shionogi and Chiesi, outside the submitted work. S.K. Field has served on advisory boards for GSK and Merck, has given sponsored talks for Boehringer Ingelheim, GlaxoSmithKline and Novartis, and has received research funding from AstraZeneca, CIHR, InsMed and Novartis, outside of the submitted work. C.L. French and R.S. Irwin disclose that they are co-developers and hold the copyright of the CQLQ and have each received less than $700 in fees over the past 3 years for its use in studies. P.G. Gibson reports grants from GlaxoSmithKline, and personal fees from AstraZeneca, Chiesi, GSK, Novartis and Sanofi, outside the submitted work. P. Marsden reports an investigator-initiated grant from Merck Sharpe & Dohme Ltd outside the submitted work. W-J. Song reports grants from MSD and AstraZeneca, consulting fees from MSD and AstraZeneca, and lecture fees from MSD, AstraZeneca, GlaxoSmithKline and Novartis, outside the submitted work. J.A. Smith reports grants from Merck, Ario Pharma, GlaxoSmithKline, NeRRe Pharmaceuticals, Menlo, Bellus and Bayer, and personal fees from Chiesi, Ario Pharma, GlaxoSmithKline, NeRRe Pharmaceuticals, Menlo, Bellus, Bayer, Boehringer Ingleheim, Genentech and Neomed, outside of the submitted work. J.A. Smith is a named inventor on a patent, owned by Manchester University NHS Foundation Trust and licensed to Vitalograph Ltd, describing the detection of cough from sound recordings. The VitaloJAK cough monitoring algorithm has been licensed by Manchester University Foundation Trust (MFT) and the University of Manchester to Vitalograph Ltd and Vitalograph Ireland (Ltd). MFT receives royalties that may be shared with the clinical division in which J.A. Smith works. P.M. O'Byrne reports grants and personal fees from AstraZeneca and Medimmune, personal fees from GlaxoSmithKline and Chiesi, and grants from Novartis and Biohaven, outside the submitted work. I. Satia reports an ERS Respire 3 Marie Curie Fellowship, grants and personal fees from Merck Canada, and personal fees from GlaxoSmithKline and AstraZeneca, outside the submitted work.
Figures
References
LinkOut - more resources
Full Text Sources
Research Materials