Light Triggered Enhancement of Antibiotic Efficacy in Biofilm Elimination Mediated by Gold-Silver Alloy Nanoparticles
- PMID: 35295305
- PMCID: PMC8919054
- DOI: 10.3389/fmicb.2022.841124
Light Triggered Enhancement of Antibiotic Efficacy in Biofilm Elimination Mediated by Gold-Silver Alloy Nanoparticles
Abstract
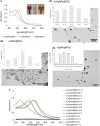
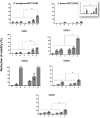
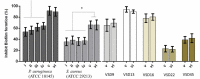
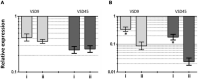
Bacterial biofilm is a tri-dimensional complex community of cells at different metabolic stages involved in a matrix of self-produced extracellular polymeric substances. Biofilm formation is part of a defense mechanism that allows the bacteria to survive in hostile environments, such as increasing resistance or tolerance to antimicrobial agents, causing persistent infections hard to treat and impair disease eradication. One such example is bovine mastitis associated with Streptococcus dysgalactiae subsp. dysgalactiae (SDSD), whose worldwide health and economic impact is on the surge. As such, non-conventional nanobased approaches have been proposed as an alternative to tackle biofilm formation and to which pathogenic bacteria fail to adapt. Among these, metallic nanoparticles have gained significant attention, particularly gold and silver nanoparticles, due to their ease of synthesis and impact against microorganism growth. This study provides a proof-of-concept investigation into the use of gold-silver alloy nanoparticles (AuAgNPs) toward eradication of bacterial biofilms. Upon visible light irradiation of AuAgNPs there was considerable disturbance of the biofilms' matrix. The hindering of structural integrity of the biofilm matrix resulted in an increased permeability for entry of antibiotics, which then cause the eradication of biofilm and inhibit subsequent biofilm formation. Additionally, our results that AuAgNPs inhibited the formation of SDSD biofilms via distinct stress pathways that lead to the downregulation of two genes critical for biofilm production, namely, brpA-like encoding biofilm regulatory protein and fbpA fibronectin-binding protein A. This study provides useful information to assist the development of nanoparticle-based strategies for the active treatment of biofilm-related infections triggered by photoirradiation in the visible.
Keywords: Streptococcus; biofilm inhibition; biofilms; eradication mature biofilms; gold-silver alloy nanoparticles.
Copyright © 2022 Alves-Barroco, Rivas-García, Fernandes and Baptista.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

