Melanin Promotes Spore Production in the Rice Blast Fungus Magnaporthe oryzae
- PMID: 35295315
- PMCID: PMC8920546
- DOI: 10.3389/fmicb.2022.843838
Melanin Promotes Spore Production in the Rice Blast Fungus Magnaporthe oryzae
Abstract
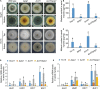
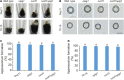
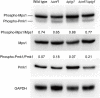
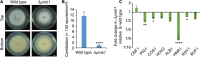
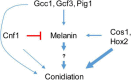
The rice blast pathogen, Magnaporthe oryzae, spreads through spores and invades rice through appressoria. Melanin is necessary for an appressorium to penetrate plant cells, but there are many unknown aspects of its role in fungal conidiation. In this study, we confirmed that melanin promotes spore production in M. oryzae, and that this effect is related to the background melanin content of wild-type strains. In the wild-type 70-15 strain with low melanin content of aerial hyphae, increased melanin synthesis promoted sporulation. In contrast, increased melanin synthesis in the wild-type Guy11 strain, which has higher melanin content, did not promote sporulation. The transcription factor Cnf1 (conidial production negative regulatory factor 1), which negatively regulates melanin synthesis, has opposite effects in conidiophore differentiation of Guy11 and 70-15. Deletion of CNF1 did not abolish the defects of Δcos1 and Δhox2 (where COS1/conidiophore stalk-less 1 or HOX2/homeodomain protein 2 was deleted) in conidiation, while increased the conidiation of Δgcc1 and Δgcf3 (where GCC1/growth, conidiation and cell wall regulatory factor 1, or GCF3/growth and conidiation regulatory factor 3 was deleted). Pig1 (pigment of Magnaporthe 1) regulates the melanin synthesis of hyphae but not of conidiophores, spores, or appressoria. Deletion of the same gene in different wild-type strains can lead to different phenotypes, partly because of differences in melanin content between fungal strains. Overall, this study reveals the functional diversity and complexity of melanin in different M. oryzae strains.
Keywords: Magnaporthe oryzae; conidia; melanin; rice blast; sporulation; transcription factor.
Copyright © 2022 Huang, Cao, Li, Zhu, Wang, Wang, Liu, Lin and Lu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Chao C. T., Ellingboe A. H. (1991). Selection for mating competence in Magnaporthe grisea pathogenic to rice. Can. J. Bot. 69 2130–2134. 10.1139/b91-267 - DOI
-
- Chumley F., Valent B. (1990). Genetic analysis of melanin-deficient, nonpathogenic mutants of Magnaporthe grisea. Mol. Plant Microbe Interact. 3:135. 10.1094/mpmi-3-135 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

