Sex Differences in a Rat Model of Peripheral Neuropathic Pain and Associated Levels of Endogenous Cannabinoid Ligands
- PMID: 35295501
- PMCID: PMC8915733
- DOI: 10.3389/fpain.2021.673638
Sex Differences in a Rat Model of Peripheral Neuropathic Pain and Associated Levels of Endogenous Cannabinoid Ligands
Abstract
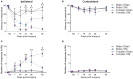
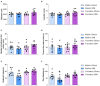
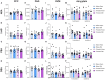
Chronic neuropathic pain is a major unmet clinical need affecting 10% of the world population, the majority of whom suffer from co-morbid mood disorders. Sex differences have been reported in pain prevalence, perception and response to analgesics. However, sexual dimorphism in chronic neuropathic pain and the associated neurobiology, are still poorly understood. The lack of efficacy and the adverse effects associated with current pharmacological treatments, further underline the need for new therapeutic targets. The endocannabinoid system (ECS) is a lipid signalling system which regulates a large number of physiological processes, including pain. The aim of this study was to investigate sexual dimorphism in pain-, anxiety- and depression-related behaviours, and concomitant alterations in supraspinal and spinal endocannabinoid levels in the spared nerve injury (SNI) animal model of peripheral neuropathic pain. Sham or SNI surgery was performed in adult male and female Sprague-Dawley rats. Mechanical and cold allodynia was tested weekly using von Frey and acetone drop tests, respectively. Development of depression-related behaviours was analysed using sucrose splash and sucrose preference tests. Locomotor activity and anxiety-related behaviours were assessed with open field and elevated plus maze tests. Levels of endocannabinoid ligands and related N-acylethanolamines in supraspinal regions of the descending inhibitory pain pathway, and spinal cord, were analysed 42 days post-surgery. SNI surgery induced allodynia in rats of both sexes. Female-SNI rats exhibited earlier onset and greater sensitivity to cold and mechanical allodynia than their male counterparts. In male rats, SNI induced a significant reduction of rearing, compared to sham controls. Trends for depressive-like behaviours in females and for anxiety-like behaviours in males were observed after SNI surgery but did not reach statistical significance. No concomitant alterations in levels of endogenous cannabinoid ligands and related N-acylethanolamines were observed in the regions analysed. Our results demonstrate differential development of SNI-induced nociceptive behaviour between male and female rats suggesting important sexually dimorphic modifications in pain pathways. SNI had no effect on depression- or anxiety-related behaviours in animals of either sex, or on levels of endocannabinoid ligands and related N-acylethanolamines across the regions involved in the descending modulation of nociception at the time points investigated.
Keywords: anxiety; chronic neuropathic pain; depression; endogenous cannabinoid ligands; sexual dimorphism; spared nerve injury.
Copyright © 2021 Boullon, Finn and Llorente-Berzal.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

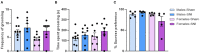
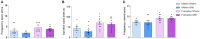
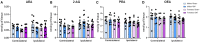
Similar articles
-
Characterisation of the effects of the chemotherapeutic agent paclitaxel on neuropathic pain-related behaviour, anxiodepressive behaviour, cognition, and the endocannabinoid system in male and female rats.Front Pharmacol. 2025 Jan 3;15:1505980. doi: 10.3389/fphar.2024.1505980. eCollection 2024. Front Pharmacol. 2025. PMID: 39830350 Free PMC article.
-
Amylin, a peptide expressed by nociceptors, modulates chronic neuropathic pain.Eur J Pain. 2019 Apr;23(4):784-799. doi: 10.1002/ejp.1347. Epub 2019 Jan 9. Eur J Pain. 2019. PMID: 30506955
-
Sex differences in the affective-cognitive dimension of neuropathic pain: Insights from the spared nerve injury rat model.J Pain. 2025 Feb;27:104752. doi: 10.1016/j.jpain.2024.104752. Epub 2024 Dec 1. J Pain. 2025. PMID: 39626836
-
The endocannabinoid system in the brain undergoes long-lasting changes following neuropathic pain.iScience. 2024 Nov 22;27(12):111409. doi: 10.1016/j.isci.2024.111409. eCollection 2024 Dec 20. iScience. 2024. PMID: 39717086 Free PMC article. Review.
-
Sexual Dimorphism in the Mechanism of Pain Central Sensitization.Cells. 2023 Aug 9;12(16):2028. doi: 10.3390/cells12162028. Cells. 2023. PMID: 37626838 Free PMC article. Review.
Cited by
-
Time-Course of Alterations in the Endocannabinoid System after Viral-Mediated Overexpression of α-Synuclein in the Rat Brain.Molecules. 2022 Jan 14;27(2):507. doi: 10.3390/molecules27020507. Molecules. 2022. PMID: 35056822 Free PMC article.
-
An improved conflict avoidance assay reveals modality-specific differences in pain hypersensitivity across sexes.Pain. 2024 Jun 1;165(6):1304-1316. doi: 10.1097/j.pain.0000000000003132. Epub 2024 Jan 26. Pain. 2024. PMID: 38277178 Free PMC article.
-
Comparison of Thermal and Mechanical Pain Testing Modalities in Sprague Dawley and Fischer 344 Rats (Rattus norvegicus).Comp Med. 2024 Jun 1;74(3):173-178. doi: 10.30802/AALAS-CM-24050. Comp Med. 2024. PMID: 39107939 Free PMC article.
-
Radial nerve injury causes long-lasting forelimb sensory impairment and motor dysfunction in rats.Pain Rep. 2021 Sep 16;6(3):e957. doi: 10.1097/PR9.0000000000000957. eCollection 2021 Sep-Oct. Pain Rep. 2021. PMID: 35187377 Free PMC article.
-
Expression and Kinetics of Endogenous Cannabinoids in the Brain and Spinal Cord of a Spare Nerve Injury (SNI) Model of Neuropathic Pain.Cells. 2022 Dec 19;11(24):4130. doi: 10.3390/cells11244130. Cells. 2022. PMID: 36552893 Free PMC article.
References
LinkOut - more resources
Full Text Sources

