Characterization of Antennal Chemosensilla and Associated Chemosensory Genes in the Orange Spiny Whitefly, Aleurocanthus spiniferus (Quaintanca)
- PMID: 35295577
- PMCID: PMC8920487
- DOI: 10.3389/fphys.2022.847895
Characterization of Antennal Chemosensilla and Associated Chemosensory Genes in the Orange Spiny Whitefly, Aleurocanthus spiniferus (Quaintanca)
Abstract
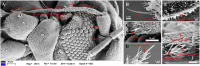
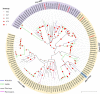
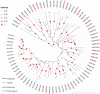
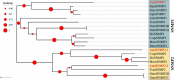
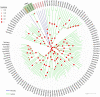
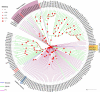
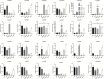
The insect chemosensory system plays an important role in many aspects of insects' behaviors necessary for their survival. Despite the complexity of this system, an increasing number of studies have begun to understand its structure and function in different insect species. Nonetheless, the chemosensory system in the orange spiny whitefly Aleurocanthus spiniferus, as one of the most destructive insect pests of citrus in tropical Asia, has not been investigated yet. In this study, the sensillum types, morphologies and distributions of the male and female antennae of A. spiniferus were characterized using scanning electron microscopy. In both sexes, six different sensilla types were observed: trichodea sensilla, chaetica sensilla, microtrichia sensilla, coeloconic sensilla, basiconic sensilla, and finger-like sensilla. Moreover, we identified a total of 48 chemosensory genes, including 5 odorant-binding proteins (OBPs), 12 chemosensory proteins (CSPs), 3 sensory neuron membrane proteins (SNMPs), 6 odorant receptors (ORs), 8 gustatory receptors (GRs), and 14 ionotropic receptors (IRs) using transcriptome data analysis. Tissue-specific transcriptome analysis of these genes showed predominantly expression in the head (including antennae), whereas CSPs were broadly expressed in both head (including the antennae) and body tissue of adult A. spiniferus. In addition, the expression profiling of selected chemosensory genes at different developmental stages was examined by quantitative real time-PCR which was mapped to the transcriptome. We found that the majority of these genes were highly expressed in adults, while AspiORco, AspiGR1, AspiGR2, and AspiIR4 genes were only detected in the pupal stage. Together, this study provides a basis for future chemosensory and genomic studies in A. spiniferus and closely related species. Furthermore, this study not only provides insights for further research on the molecular mechanisms of A. spiniferus-plant interactions but also provides extensive potential targets for pest control.
Keywords: Aleurocanthus spiniferus; antennal sensilla; chemosensory genes; expression patterns; transcriptome.
Copyright © 2022 Gao, Chen, Liu, Song, Jia, Liu, Qu, Dewer, Zhao, Xu and Kang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

