Bullous Pemphigoid Associated With COVID-19 Vaccines: An Italian Multicentre Study
- PMID: 35295599
- PMCID: PMC8918943
- DOI: 10.3389/fmed.2022.841506
Bullous Pemphigoid Associated With COVID-19 Vaccines: An Italian Multicentre Study
Abstract
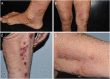
Bullous pemphigoid (BP) is an autoimmune bullous disease caused by circulating autoantibodies toward the hemidesmosomal antigens BP180 and BP230. Cases of BP have been described following vaccinations against tetanus, poliomyelitis, diphtheria, influenza, pneumococcus, meningococcus, hepatitis B and rabies. The putative mechanism by which COVID-19-vaccines may induce BP has not been clarified. An Italian multicentre study was conducted to collect clinical, histopathological and immunopathological data of patients with BP associated with COVID-19-vaccines. Twenty-one cases were collected, including 9 females and 12 males (M/F = 1.3) with a median age at diagnosis of 82 years. Seventeen patients received the COMIRNATY Pfizer-BioNTech vaccine, two the Moderna mRNA-1273 vaccine, one the ChAdOx1/nCoV-19-AstraZeneca/ Vaxzevria vaccine and one received the first dose with the ChAdOx1/nCoV-19-AstraZeneca/Vaxzevria vaccine and the second dose with the COMIRNATY Pfizer-BioNTech vaccine. Median latency time between the first dose of anti-SARS-CoV-2 vaccine and the onset of cutaneous manifestations was 27 days. Median BPDAI at onset was 42. Eleven out of seventeen patients (65%) had positive titres for anti-BP180 antibodies with a median value of 106.3 U/mL on ELISA; in contrast, only five out of seventeen (29%) were positive for anti-BP230 antibodies, with a median of 35.3 U/mL. In conclusion, in terms of mean age, disease severity at diagnosis and clinical phenotype vaccine-associated BP patients seem to be similar to idiopathic BP with an overall benign course with appropriate treatment. On the other hand, the slight male predominance and the reduced humoral response to BP230 represent peculiar features of this subset of patients.
Keywords: BP180; BP230; COVID-19; SARS-CoV-2; autoantibodies; bullous pemphigoid; triggering factors; vaccine.
Copyright © 2022 Maronese, Caproni, Moltrasio, Genovese, Vezzoli, Sena, Previtali, Cozzani, Gasparini, Parodi, Atzori, Antiga, Maglie, Moro, Mariotti, Corrà, Pallotta, Didona, Marzano and Di Zenzo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Comment in
-
Commentary: Bullous pemphigoid associated with COVID-19 vaccines: An Italian multicenter study.Front Med (Lausanne). 2022 Dec 19;9:1096867. doi: 10.3389/fmed.2022.1096867. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36600888 Free PMC article. No abstract available.
References
-
- Chacón GR, Sinha AA. Bullous pemphigoid after herpes zoster vaccine administration: association or coincidence? J Drugs Dermatol. (2011) 10:1328-30. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

