Anti-PD-L1 Antibody Enhances T Cell Immune Responses and Reduces Resistance of Breast Cancer Cells to Radiotherapy
- PMID: 35295718
- PMCID: PMC8920704
- DOI: 10.1155/2022/5938688
Anti-PD-L1 Antibody Enhances T Cell Immune Responses and Reduces Resistance of Breast Cancer Cells to Radiotherapy
Abstract
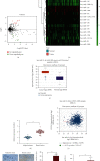
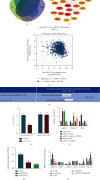
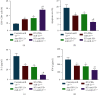
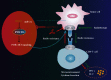
Immune escape is a frequent occurrence, which limits the duration of antitumor immune responses to radiotherapy. Here, we aimed to ascertain the roles and underlying mechanisms of programmed death ligand 1 (PD-L1) in tolerance of breast cancer (BC) to radiotherapy. We first quantified microRNA-21 (miR-21) and PD-L1 expression in BC tissues and cells, followed by identification of the interactions between miR-21, PD-L1, and programmed cell death protein 4 (PDCD4). miR-21 knock-in mice were used to construct tumor-bearing models, which were then treated with anti-PD-L1 antibody and irradiation, followed by measurement of tumor growth and tumor immune escape. Finally, we evaluated the synergistic effects of radiotherapy and anti-PD-L1 antibody in vivo. The results showed increased miR-21 expression in BC tissues and cells, which was positively correlated with PD-L1 expression. The treatment with radiotherapy or anti-PD-L1 antibody in the miR-21 knock-in mice diminished tumor weight and volume, along with decreased CD3+CD8+ positive cells, serum IL-2 and IFN-γ levels, and lower PD-L1 expression, but augmented apoptosis of T and BC cells. Moreover, miR-21 significantly augmented PD-L1 expression via PI3K/Akt pathway activation by targeting PDCD4 in BC cells. Thus, radiotherapy and anti-PD-L1 antibody synergistically accelerated the therapeutic effect against BC in mice, thereby implicating a close interplay between radiotherapy, T cells, and the miR-21/PDCD4/PI3K/Akt/PD-L1 axis.
Copyright © 2022 Lei-Ming Guo et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

