β-Sitosterol Inhibits Rheumatoid Synovial Angiogenesis Through Suppressing VEGF Signaling Pathway
- PMID: 35295740
- PMCID: PMC8918576
- DOI: 10.3389/fphar.2021.816477
β-Sitosterol Inhibits Rheumatoid Synovial Angiogenesis Through Suppressing VEGF Signaling Pathway
Abstract
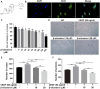
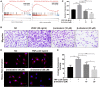
Background: Rheumatoid arthritis (RA) is a chronic disabling inflammatory disease that causes synovial angiogenesis in an invasive manner and leads to joint destruction. Currently available pharmacotherapy for RA has unwanted side effects and limitations. Although anti-angiogenic therapy is regarded as a new potential treatment for RA, only a few anti-angiogenic drugs are available. An increasing number of studies have shown that β-sitosterol (BSS) may exert inhibitory effects against angiogenesis. However, the mechanisms involved are still unclear. Methods: Based on the results of the gene set enrichment analysis (GSEA) of the transcriptome data of endothelial cells from RA patients, we evaluated the pharmacological effects of BSS on the tube formation, cell proliferation, and migration of human umbilical vein endothelial cells (HUVECs). Furthermore, the effects of BSS treatment on vascular endothelial growth factor receptor 2 (VEGFR2) were determined using molecular docking and Western blotting. Additionally, in the presence or absence of BSS, synovial angiogenesis and joint destruction of the ankle were investigated in collagen-induced arthritis (CIA) mice. The effect of BSS treatment on VEGFR2/p-VEGFR2 expression was verified through immunohistochemical staining. Results: The immunohistochemistry results revealed that BSS treatment inhibited angiogenesis both in vitro and in vivo. In addition, the results of 5-ethynyl-2'-deoxyuridine and cell cycle analysis showed that BSS treatment suppressed the proliferation of HUVECs, while the Transwell migration and stress fiber assays demonstrated that BSS treatment inhibited the migration of HUVECs. Notably, the inhibitory effect of BSS treatment on VEGFR2/p-VEGFR2 was similar to that of axitinib. In CIA mice, BSS also exerted therapeutic effects on the ankles by reducing the degree of swelling, ameliorating bone and cartilage damage, preventing synovial angiogenesis, and inhibiting VEGFR2 and p-VEGFR2 expression. Conclusion: Therefore, our findings demonstrate that BSS exerts an inhibitory effect on synovial angiogenesis by suppressing the proliferation and migration of endothelial cells, thereby alleviating joint swelling and bone destruction in CIA mice. Furthermore, the underlying therapeutic mechanisms may involve the inhibition of VEGF signaling pathway activation.
Keywords: VEGFR2; angiogenesis; collagen-induced arthritis; endothelial cells; rheumatoid arthritis; β-sitosterol.
Copyright © 2022 Qian, Zheng, Wang, Huang, Liu, Xu, Liu, Liu and Lin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures