Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Meta-Analysis of Randomized Controlled Trials
- PMID: 35295757
- PMCID: PMC8919053
- DOI: 10.3389/fcimb.2022.827395
Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Meta-Analysis of Randomized Controlled Trials
Abstract
Background: Randomized controlled trials (RCTs) have examined the efficacy of fecal microbiota transplantation (FMT) in irritable bowel syndrome (IBS) with inconsistent results. We performed a meta-analysis to assess both the short- and long-term efficacy of FMT in IBS.
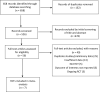
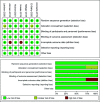
Methods: MEDLINE, EMBASE, and the Cochrane Central Register were searched through September 2021. RCTs recruiting adult patients with IBS that compared FMT with placebo with dichotomous data of response to therapy were eligible. Dichotomous data were pooled to obtain a relative risk (RR) of symptom not improving after therapy. RR was also pooled for adverse events (AEs). Continuous data were calculated using a mean difference for IBS-Quality of Life (IBS-QoL). GRADE methodology was used to assess quality of evidence.
Results: The search strategy generated 658 citations. Seven RCTs comprising 472 patients with IBS were included. FMT was not associated with a significant improvement in global symptom in IBS at 12 weeks in comparison with placebo (RR 0.75, 95% CI 0.43-1.31) with high heterogeneity between studies (I2 87%). Subgroup analyses showed that FMT was superior to placebo when administered via colonoscopy or gastroscope (RR 0.70, 95% CI 0.51-0.96; RR 0.37, 95% CI 0.14-0.99, respectively, while FMT was inferior to placebo when administered via oral capsules (RR 1.88, 95% CI 1.06-3.35). FMT induced a significant improvement in IBS-QoL compared to placebo (mean difference 9.39, 95% CI 3.86-14.91) at 12 weeks. No significant difference in the total number of AEs was observed between FMT and placebo (RR 1.20, 95% CI 0.59-2.47). FMT did not significantly improve global symptom in IBS at 1-year follow-up compared with placebo (RR 0.90, 95% CI 0.72-1.12). The GRADE quality evidence to support recommending FMT in IBS was very low.
Conclusion: IBS patients may benefit from FMT when administered via colonoscopy or gastroscope. FMT may improve the quality of life of IBS. The long-term use of FMT in IBS warrants further investigation. There is very-low-quality evidence to support recommending FMT in IBS.
Keywords: fecal microbiota transplantation; intestinal microbiota; irritable bowel syndrome; meta-analysis; microbiota.
Copyright © 2022 Wu, Lv and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

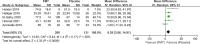

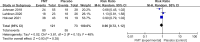
Similar articles
-
A meta-analysis of randomized controlled trials evaluating the effectiveness of fecal microbiota transplantation for patients with irritable bowel syndrome.BMC Gastroenterol. 2024 Jul 5;24(1):217. doi: 10.1186/s12876-024-03311-x. BMC Gastroenterol. 2024. PMID: 38970007 Free PMC article.
-
Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis.Am J Gastroenterol. 2019 Jul;114(7):1043-1050. doi: 10.14309/ajg.0000000000000198. Am J Gastroenterol. 2019. PMID: 30908299 Free PMC article.
-
Fecal microbiota transplantation for the treatment of irritable bowel syndrome: A systematic review and meta-analysis.World J Gastroenterol. 2023 May 28;29(20):3185-3202. doi: 10.3748/wjg.v29.i20.3185. World J Gastroenterol. 2023. PMID: 37346153 Free PMC article.
-
Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome.Aliment Pharmacol Ther. 2019 Aug;50(3):240-248. doi: 10.1111/apt.15330. Epub 2019 May 28. Aliment Pharmacol Ther. 2019. PMID: 31136009
-
Fecal microbiota transplantation for irritable bowel syndrome: a systematic review and meta-analysis of randomized controlled trials.Front Immunol. 2023 May 18;14:1136343. doi: 10.3389/fimmu.2023.1136343. eCollection 2023. Front Immunol. 2023. PMID: 37275867 Free PMC article.
Cited by
-
Clinical efficacy and safety of faecal microbiota transplantation in the treatment of irritable bowel syndrome: a systematic review, meta-analysis and trial sequential analysis.Eur J Med Res. 2024 Sep 18;29(1):464. doi: 10.1186/s40001-024-02046-5. Eur J Med Res. 2024. PMID: 39289768 Free PMC article.
-
Co-Housing and Fecal Microbiota Transplantation: Technical Support for TCM Herbal Treatment of Extra-Intestinal Diseases Based on Gut Microbial Ecosystem Remodeling.Drug Des Devel Ther. 2023 Dec 24;17:3803-3831. doi: 10.2147/DDDT.S443462. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 38155743 Free PMC article. Review.
-
Efficacy and safety of fecal microbiota transplantation for the treatment of irritable bowel syndrome: an overview of overlapping systematic reviews.Front Pharmacol. 2023 Oct 17;14:1264779. doi: 10.3389/fphar.2023.1264779. eCollection 2023. Front Pharmacol. 2023. PMID: 37915416 Free PMC article.
-
Short-Chain Fatty Acids and Human Health: From Metabolic Pathways to Current Therapeutic Implications.Life (Basel). 2024 Apr 26;14(5):559. doi: 10.3390/life14050559. Life (Basel). 2024. PMID: 38792581 Free PMC article. Review.
-
The Efficacy of Probiotics, Prebiotics, Synbiotics, and Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Systematic Review and Network Meta-Analysis.Nutrients. 2024 Jul 2;16(13):2114. doi: 10.3390/nu16132114. Nutrients. 2024. PMID: 38999862 Free PMC article.
References
-
- Aroniadis O. C., Brandt L. J., Oneto C., Feuerstadt P., Sherman A., Wolkoff A. W., et al. . (2019). Faecal Microbiota Transplantation for Diarrhoea-Predominant Irritable Bowel Syndrome: A Double-Blind, Randomised, Placebo-Controlled Trial. Lancet Gastroenterol. Hepatol. 4 (9), 675–685. doi: 10.1016/S2468-1253(19)30198-0 - DOI - PubMed
-
- Bosman M., Elsenbruch S., Corsetti M., Tack J., Simren M., Winkens B., et al. . (2021). The Placebo Response Rate in Pharmacological Trials in Patients With Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 6 (6), 459–473. doi: 10.1016/S2468-1253(21)00023-6 - DOI - PubMed
-
- Carroll I. M., Ringel-Kulka T., Siddle J. P., Ringel Y. (2012). Alterations in Composition and Diversity of the Intestinal Microbiota in Patients With Diarrhea-Predominant Irritable Bowel Syndrome. Neurogastroenterol. Motil. 24 (6), 521–530, e248. doi: 10.1111/j.1365-2982.2012.01891.x - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

