Reduced Hippocampal Volume and Neurochemical Response to Adult Stress Exposure in a Female Mouse Model of Urogenital Hypersensitivity
- PMID: 35295799
- PMCID: PMC8915737
- DOI: 10.3389/fpain.2022.809944
Reduced Hippocampal Volume and Neurochemical Response to Adult Stress Exposure in a Female Mouse Model of Urogenital Hypersensitivity
Abstract
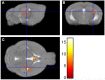
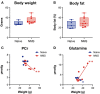
Early life stress exposure significantly increases the risk of developing chronic pain syndromes and comorbid mood and metabolic disorders later in life. Structural and functional changes within the hippocampus have been shown to contribute to many early life stress-related outcomes. We have previously reported that adult mice that underwent neonatal maternal separation (NMS) exhibit urogenital hypersensitivity, altered anxiety- and depression-like behaviors, increased adiposity, and decreased gene expression and neurogenesis in the hippocampus. Here, we are using magnetic resonance imaging and spectroscopy (MRI and MRS) to further investigate both NMS- and acute stress-induced changes in the hippocampus of female mice. Volumetric analysis of the whole brain revealed that the left hippocampus of NMS mice was 0.038 mm3 smaller compared to naïve mice. MRS was performed only on the right hippocampus and both total choline (tCho) and total N-acetylaspartate (tNAA) levels were significantly decreased due to NMS, particularly after WAS. Phosphoethanolamine (PE) levels were decreased in naïve mice after WAS, but not in NMS mice, and WAS increased ascorbate levels in both groups. The NMS mice showed a trend toward increased body weight and body fat percentage compared to naïve mice. A significant negative correlation was observed between body weight and phosphocreatine levels post-WAS in NMS mice, as well as a positive correlation between body weight and glutamine for NMS mice and a negative correlation for naïve mice. Together, these data suggest that NMS in mice reduces left hippocampal volume and may result in mitochondrial dysfunction and reduced neuronal integrity of the right hippocampus in adulthood. Hippocampal changes also appear to be related to whole body metabolic outcomes.
Keywords: early life stress; magnetic resonance imaging; magnetic resonance spectroscopy; obesity; pain.
Copyright © 2022 Brake, Yang, Lee, Lee, Keselman, Eller, Choi, Harris and Christianson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Child Adolescent Health Measurement Initiative . U.S. Department of Health and Human Services, Health Resources and Services Administration (HRSA), Maternal and Child Health Bureau (MCHB) (2018).
Grants and funding
LinkOut - more resources
Full Text Sources

