The role of long noncoding RNA Nron in atherosclerosis development and plaque stability
- PMID: 35295812
- PMCID: PMC8919297
- DOI: 10.1016/j.isci.2022.103978
The role of long noncoding RNA Nron in atherosclerosis development and plaque stability
Abstract
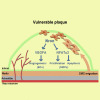
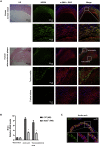
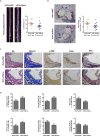
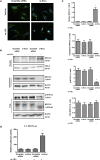
The major clinical consequences of atherosclerosis such as myocardial infarction or stroke are because of thrombotic events associated with acute rupture or erosion of an unstable plaque. Here, we identify an lncRNA Noncoding Repressor of NFAT (Nron) as a critical regulator of atherosclerotic plaque stability. Nron overexpression (OE) in vascular smooth muscle cells (VSMC) induces a highly characteristic architecture of more-vulnerable plaques, while Nron knockdown (KD) suppresses the development of atherosclerosis and favors plaque stability. Mechanistically, Nron specifically binds to and negatively regulates NFATc3, thus inhibiting the proliferation and promoting the apoptosis of VSMCs. Moreover, we also provide evidence that Nron increases the production and secretion of VEGFA from VSMCs, which functions as a paracrine factor to enhance intra-plaque angiogenesis. All of these effects contribute to plaque instability. Genetic or pharmacological inhibition of Nron may have potential for future therapy of atherosclerosis.
Keywords: Functional aspects of cell biology; Molecular biology; Pathophysiology.
© 2022 The Authors.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures





Similar articles
-
Genetic Evidence Supports a Major Role for Akt1 in VSMCs During Atherogenesis.Circ Res. 2015 May 22;116(11):1744-52. doi: 10.1161/CIRCRESAHA.116.305895. Epub 2015 Apr 13. Circ Res. 2015. PMID: 25868464 Free PMC article.
-
Potential role of insulin receptor isoforms and IGF receptors in plaque instability of human and experimental atherosclerosis.Cardiovasc Diabetol. 2018 Feb 20;17(1):31. doi: 10.1186/s12933-018-0675-2. Cardiovasc Diabetol. 2018. PMID: 29463262 Free PMC article.
-
LncRNA NRON alleviates atrial fibrosis through suppression of M1 macrophages activated by atrial myocytes.Biosci Rep. 2019 Nov 29;39(11):BSR20192215. doi: 10.1042/BSR20192215. Biosci Rep. 2019. PMID: 31693733 Free PMC article.
-
The emerging role of vascular smooth muscle cell apoptosis in atherosclerosis and plaque stability.Am J Nephrol. 2006;26(6):531-5. doi: 10.1159/000097815. Epub 2006 Dec 6. Am J Nephrol. 2006. PMID: 17159340 Review.
-
Role of apoptosis in atherosclerosis and its therapeutic implications.Clin Sci (Lond). 2004 Oct;107(4):343-54. doi: 10.1042/CS20040086. Clin Sci (Lond). 2004. PMID: 15230690 Review.
Cited by
-
LncRNA MDRL Mitigates Atherosclerosis through miR-361/SQSTM1/NLRP3 Signaling.Mediators Inflamm. 2022 Sep 21;2022:5463505. doi: 10.1155/2022/5463505. eCollection 2022. Mediators Inflamm. 2022. PMID: 36186576 Free PMC article.
-
Noncoding RNAs in atherosclerosis: regulation and therapeutic potential.Mol Cell Biochem. 2024 May;479(5):1279-1295. doi: 10.1007/s11010-023-04794-0. Epub 2023 Jul 7. Mol Cell Biochem. 2024. PMID: 37418054 Free PMC article. Review.
-
The role of long non-coding RNAs in cardiovascular diseases: A comprehensive review.Noncoding RNA Res. 2024 Dec 28;11:158-187. doi: 10.1016/j.ncrna.2024.12.009. eCollection 2025 Apr. Noncoding RNA Res. 2024. PMID: 39896344 Free PMC article. Review.
-
LncRNA NRON promotes tumorigenesis by enhancing MDM2 activity toward tumor suppressor substrates.EMBO J. 2023 Aug 15;42(16):e112414. doi: 10.15252/embj.2022112414. Epub 2023 Jun 29. EMBO J. 2023. PMID: 37382239 Free PMC article.
-
lncRNA PCA3 Suppressed Carotid Artery Stenosis and Vascular Smooth Muscle Cell Function via Negatively Modulating the miR-124-3p/ITGB1 Axis.Clin Appl Thromb Hemost. 2023 Jan-Dec;29:10760296231190383. doi: 10.1177/10760296231190383. Clin Appl Thromb Hemost. 2023. PMID: 37583257 Free PMC article.
References
-
- de Nooijer R., Bot I., von der Thusen J.H., Leeuwenburgh M.A., Overkleeft H.S., Kraaijeveld A.O., Dorland R., van Santbrink P.J., van Heiningen S.H., Westra M.M., et al. Leukocyte cathepsin S is a potent regulator of both cell and matrix turnover in advanced atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009;29:188–194. doi: 10.1161/ATVBAHA.108.181578. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

