Efficacy and Safety of Regdanvimab (CT-P59): A Phase 2/3 Randomized, Double-Blind, Placebo-Controlled Trial in Outpatients With Mild-to-Moderate Coronavirus Disease 2019
- PMID: 35295819
- PMCID: PMC8903348
- DOI: 10.1093/ofid/ofac053
Efficacy and Safety of Regdanvimab (CT-P59): A Phase 2/3 Randomized, Double-Blind, Placebo-Controlled Trial in Outpatients With Mild-to-Moderate Coronavirus Disease 2019
Abstract
Background: Regdanvimab (CT-P59) is a monoclonal antibody with neutralizing activity against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). We report on part 1 of a 2-part randomized, placebo-controlled, double-blind study for patients with mild-to-moderate coronavirus disease 2019 (COVID-19).
Methods: Outpatients with mild-to-moderate COVID-19 received a single dose of regdanvimab 40 mg/kg (n = 100), regdanvimab 80 mg/kg (n = 103), or placebo (n = 104). The primary end points were time to negative conversion of SARS-CoV-2 from nasopharyngeal swab based on quantitative reverse transcription polymerase chain reaction (RT-qPCR) up to day 28 and time to clinical recovery up to day 14. Secondary end points included the proportion of patients requiring hospitalization, oxygen therapy, or mortality due to COVID-19.
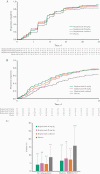
Results: Median (95% CI) time to negative conversion of RT-qPCR was 12.8 (9.0-12.9) days with regdanvimab 40 mg/kg, 11.9 (8.9-12.9) days with regdanvimab 80 mg/kg, and 12.9 (12.7-13.9) days with placebo. Median (95% CI) time to clinical recovery was 5.3 (4.0-6.8) days with regdanvimab 40 mg/kg, 6.2 (5.5-7.9) days with regdanvimab 80 mg/kg, and 8.8 (6.8-11.6) days with placebo. The proportion (95% CI) of patients requiring hospitalization or oxygen therapy was lower with regdanvimab 40 mg/kg (4.0% [1.6%-9.8%]) and regdanvimab 80 mg/kg (4.9% [2.1%-10.9%]) vs placebo (8.7% [4.6%-15.6%]). No serious treatment-emergent adverse events or deaths occurred.
Conclusions: Regdanvimab showed a trend toward a minor decrease in time to negative conversion of RT-qPCR results compared with placebo and reduced the need for hospitalization and oxygen therapy in patients with mild-to-moderate COVID-19.
Clinical trial registration : NCT04602000 and EudraCT 2020-003369-20.
Keywords: 19; COVID; CT; P59; SARS-CoV-2; regdanvimab.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures
References
-
- World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Available at: https://covid19.who.int/. Accessed 17 May 2021.
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

