Antibody-Drug Conjugates Targeting the Human Epidermal Growth Factor Receptor Family in Cancers
- PMID: 35295841
- PMCID: PMC8919033
- DOI: 10.3389/fmolb.2022.847835
Antibody-Drug Conjugates Targeting the Human Epidermal Growth Factor Receptor Family in Cancers
Abstract
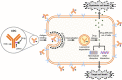
Members of the human epidermal growth factor receptor (HER) family, which includes HER1 (also known as EGFR), HER2, HER3 and HER4, have played a central role in regulating cell proliferation, survival, differentiation and migration. The overexpression of the HER family has been recognized as one of the most common cellular dysregulation associated with a wide variety of tumor types. Antibody-drug conjugates (ADCs) represent a new and promising class of anticancer therapeutics that combine the cancer specificity of antibodies with cytotoxicity of chemotherapeutic drugs. Two HER2-directed ADCs, trastuzumane-emtansine (T-DM1) and trastuzumab-deruxtecan (DS-8201a), have been approved for HER2-positive metastatic breast cancer by the U.S. Food and Drug Administration (FDA) in 2013 and 2019, respectively. A third HER2-directed ADC, disitamab vedotin (RC48), has been approved for locally advanced or metastatic gastric or gastroesophageal junction cancer by the NMPA (National Medical Products Administration) of China in 2021. A total of 11 ADCs that target HER family receptors (EGFR, HER2 or HER3) are currently under clinical trials. In this review article, we summarize the three approved ADCs (T-DM1, DS-8201a and RC48), together with the investigational EGFR-directed ADCs (ABT-414, MRG003 and M1231), HER2-directed ADCs (SYD985, ARX-788, A166, MRG002, ALT-P7, GQ1001 and SBT6050) and HER3-directed ADC (U3-1402). Lastly, we discuss the major challenges associated with the development of ADCs, and highlight the possible future directions to tackle these challenges.
Keywords: EGFR; HER family; HER2; HER3; antibody-drug conjugates; cancer targeted therapy; drug resistance.
Copyright © 2022 Yu, Fang, Yun, Liu and Cai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Banerji U., van Herpen C. M. L., Saura C., Thistlethwaite F., Lord S., Moreno V., et al. (2019). Trastuzumab Duocarmazine in Locally Advanced and Metastatic Solid Tumours and HER2-Expressing Breast Cancer: a Phase 1 Dose-Escalation and Dose-Expansion Study. Lancet Oncol. 20 (8), 1124–1135. 10.1016/S1470-2045(19)30328-6 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous