MicroRNA-210 Controls Mitochondrial Metabolism and Protects Heart Function in Myocardial Infarction
- PMID: 35296158
- PMCID: PMC9007902
- DOI: 10.1161/CIRCULATIONAHA.121.056929
MicroRNA-210 Controls Mitochondrial Metabolism and Protects Heart Function in Myocardial Infarction
Abstract
Background: Ischemic heart disease remains a leading cause of death worldwide. In this study, we test the hypothesis that microRNA-210 protects the heart from myocardial ischemia-reperfusion (IR) injury by controlling mitochondrial bioenergetics and reactive oxygen species (ROS) flux.
Methods: Myocardial infarction in an acute setting of IR was examined through comparing loss- versus gain-of-function experiments in microRNA-210-deficient and wild-type mice. Cardiac function was evaluated by echocardiography. Myocardial mitochondria bioenergetics was examined using a Seahorse XF24 Analyzer.
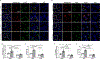
Results: MicroRNA-210 deficiency significantly exaggerated cardiac dysfunction up to 6 weeks after myocardial IR in male, but not female, mice. Intravenous injection of microRNA-210 mimic blocked the effect and recovered the increased myocardial IR injury and cardiac dysfunction. Analysis of mitochondrial metabolism revealed that microRNA-210 inhibited mitochondrial oxygen consumption, increased glycolytic activity, and reduced mitochondrial ROS flux in the heart during IR injury. Inhibition of mitochondrial ROS with MitoQ consistently reversed the effect of microRNA-210 deficiency. Mechanistically, we showed that mitochondrial glycerol-3-phosphate dehydrogenase is a novel target of microRNA-210 in the heart, and loss-of-function and gain-of-function experiments revealed that glycerol-3-phosphate dehydrogenase played a key role in the microRNA-210-mediated effect on mitochondrial metabolism and ROS flux in the setting of heart IR injury. Knockdown of glycerol-3-phosphate dehydrogenase negated microRNA-210 deficiency-induced increases in mitochondrial ROS production and myocardial infarction and improved left ventricular fractional shortening and ejection fraction after the IR treatment.
Conclusions: MicroRNA-210 targeting glycerol-3-phosphate dehydrogenase controls mitochondrial bioenergetics and ROS flux and improves cardiac function in a murine model of myocardial infarction in the setting of IR injury. The findings suggest new insights into the mechanisms and therapeutic targets for treatment of ischemic heart disease.
Keywords: energy metabolism; glycerolphosphate dehydrogenase; microRNA-210; myocardial infarction.
Figures


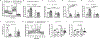

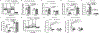


Comment in
-
Response by Song and Zhang to Letters Regarding Article, "MicroRNA-210 Controls Mitochondrial Metabolism and Protects Heart Function in Myocardial Infarction".Circulation. 2022 Sep 20;146(12):e171-e172. doi: 10.1161/CIRCULATIONAHA.122.061220. Epub 2022 Sep 19. Circulation. 2022. PMID: 36121909 Free PMC article. No abstract available.
-
Letter by De et al Regarding Article, "MicroRNA-210 Controls Mitochondrial Metabolism and Protects Heart Function in Myocardial Infarction".Circulation. 2022 Sep 20;146(12):e170. doi: 10.1161/CIRCULATIONAHA.122.060858. Epub 2022 Sep 19. Circulation. 2022. PMID: 36121910 No abstract available.
-
Letter by Hou et al Regarding Article, "MicroRNA-210 Controls Mitochondrial Metabolism and Protects Heart Function in Myocardial Infarction".Circulation. 2022 Sep 20;146(12):e169. doi: 10.1161/CIRCULATIONAHA.122.060406. Epub 2022 Sep 19. Circulation. 2022. PMID: 36121913 No abstract available.
References
-
- Mortality GBD, Causes of Death C. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2 - DOI - PMC - PubMed
-
- Karakas M, Schulte C, Appelbaum S, Ojeda F, Lackner KJ, Munzel T, Schnabel RB, Blankenberg S, Zeller T. Circulating microRNAs strongly predict cardiovascular death in patients with coronary artery disease-results from the large AtheroGene study. Eur Heart J. 2017;38:516–523. doi: 10.1093/eurheartj/ehw250 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

