Association between covid-19 vaccination, SARS-CoV-2 infection, and risk of immune mediated neurological events: population based cohort and self-controlled case series analysis
- PMID: 35296468
- PMCID: PMC8924704
- DOI: 10.1136/bmj-2021-068373
Association between covid-19 vaccination, SARS-CoV-2 infection, and risk of immune mediated neurological events: population based cohort and self-controlled case series analysis
Abstract
Objective: To study the association between covid-19 vaccines, SARS-CoV-2 infection, and risk of immune mediated neurological events.
Design: Population based historical rate comparison study and self-controlled case series analysis.
Setting: Primary care records from the United Kingdom, and primary care records from Spain linked to hospital data.
Participants: 8 330 497 people who received at least one dose of covid-19 vaccines ChAdOx1 nCoV-19, BNT162b2, mRNA-1273, or Ad.26.COV2.S between the rollout of the vaccination campaigns and end of data availability (UK: 9 May 2021; Spain: 30 June 2021). The study sample also comprised a cohort of 735 870 unvaccinated individuals with a first positive reverse transcription polymerase chain reaction test result for SARS-CoV-2 from 1 September 2020, and 14 330 080 participants from the general population.
Main outcome measures: Outcomes were incidence of Bell's palsy, encephalomyelitis, Guillain-Barré syndrome, and transverse myelitis. Incidence rates were estimated in the 21 days after the first vaccine dose, 90 days after a positive test result for SARS-CoV-2, and between 2017 and 2019 for background rates in the general population cohort. Indirectly standardised incidence ratios were estimated. Adjusted incidence rate ratios were estimated from the self-controlled case series.
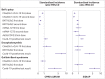
Results: The study included 4 376 535 people who received ChAdOx1 nCoV-19, 3 588 318 who received BNT162b2, 244 913 who received mRNA-1273, and 120 731 who received Ad26.CoV.2; 735 870 people with SARS-CoV-2 infection; and 14 330 080 people from the general population. Overall, post-vaccine rates were consistent with expected (background) rates for Bell's palsy, encephalomyelitis, and Guillain-Barré syndrome. Self-controlled case series was conducted only for Bell's palsy, given limited statistical power, but with no safety signal seen for those vaccinated. Rates were, however, higher than expected after SARS-CoV-2 infection. For example, in the data from the UK, the standardised incidence ratio for Bell's palsy was 1.33 (1.02 to 1.74), for encephalomyelitis was 6.89 (3.82 to 12.44), and for Guillain-Barré syndrome was 3.53 (1.83 to 6.77). Transverse myelitis was rare (<5 events in all vaccinated cohorts) and could not be analysed.
Conclusions: No safety signal was observed between covid-19 vaccines and the immune mediated neurological events of Bell's palsy, encephalomyelitis, Guillain-Barré syndrome, and transverse myelitis. An increased risk of Bell's palsy, encephalomyelitis, and Guillain-Barré syndrome was, however, observed for people with SARS-CoV-2 infection.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Contributors: XL and BR are joint first authors. DP-A, EB, and TD-S are joint senior authors. XL, BR, DP-A, TD-S, EB, and VS conceived the study and contributed to the study design. XL, BR, ER, and AP conducted the statistical analyses. XL, BR, ER, VS, DP-A, EB, and TD-S interpreted the results and wrote the manuscript. All authors contributed to writing the manuscript, approved the final version, and had final responsibility for the decision to submit for publication. TD-S, EB, and DP-A are guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. Competing interests: All authors have completed the ICMJE disclosure form at http://www.icmje.org/disclosure-of-interest/ and declare the following interests: DP-A receives funding from the UK National Institute for Health Research (NIHR) in the form of a senior research fellowship and from the Oxford NIHR Biomedical Research Centre. XL receives the Clarendon Fund and Brasenose College scholarship (University of Oxford) to support her DPhil study. DP-A’s research group has received research grants from the European Medicines Agency; the Innovative Medicines Initiative; and Amgen, Chiesi, and UCB Biopharma; and consultancy or speaker fees from Astellas, Amgen, AstraZeneca, and UCB Biopharma.
Figures

Comment in
-
The neurological safety of covid-19 vaccines.BMJ. 2022 Mar 16;376:o522. doi: 10.1136/bmj.o522. BMJ. 2022. PMID: 35296459 No abstract available.
-
COVID-19 diagnosis, but not vaccination, was linked to increased risk for immune-mediated neurologic events.Ann Intern Med. 2022 Jul;175(7):JC82. doi: 10.7326/J22-0048. Epub 2022 Jul 5. Ann Intern Med. 2022. PMID: 35785537
References
-
- WHO. COVID-19 Dashboard. Geneva: World Health Organization, 2020. https://covid19.who.int (accessed 14 January 2022).
-
- European Medicines Agency. COVID-19 vaccines: authorised. www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/cor... (accessed 14 January 2022).
-
- Voysey M, Clemens SAC, Madhi SA, et al. Oxford COVID Vaccine Trial Group . Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99-111. 10.1016/S0140-6736(20)32661-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
