Development and testing of an opioid tapering self-management intervention for chronic pain: I-WOTCH
- PMID: 35296478
- PMCID: PMC8928279
- DOI: 10.1136/bmjopen-2021-053725
Development and testing of an opioid tapering self-management intervention for chronic pain: I-WOTCH
Abstract
Objectives: To describe the design, development and pilot of a multicomponent intervention aimed at supporting withdrawal of opioids for people with chronic non-malignant pain for future evaluation in the Improving the Wellbeing of people with Opioid Treated CHronic pain (I-WOTCH) randomised controlled trial.
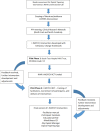
Design: The I-WOTCH intervention draws on previous literature and collaboration with stakeholders (patient and public involvement). Intervention mapping and development activities of Behaviour Change Taxonomy are described.
Setting: The intervention development was conducted by a multidisciplinary team with clinical, academic and service user perspectives. The team had expertise in the development and testing of complex health behaviour interventions, opioid tapering and pain management in primary and secondary care, I.T programming, and software development-to develop an opioid tapering App.
Participants: The I-WOTCH trial participants are adults (18 years and over) with chronic non-malignant pain using strong opioids for at least 3 months and on most days in the preceding month.
Outcomes: A multicomponent self-management support package to help people using opioids for chronic non-malignant pain reduce opioid use.
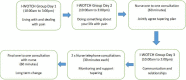
Interventions and results: Receiving information on the impact of long-term opioid use, and potential adverse effects were highlighted as important facilitators in making the decision to reduce opioids. Case studies of those who have successfully stopped taking opioids were also favoured as a facilitator to reduce opioid use. Barriers included the need for a 'trade-off to fill the deficit of the effect of the drug'. The final I-WOTCH intervention consists of an 8-10 week programme incorporating: education; problem-solving; motivation; group and one to one tailored planning; reflection and monitoring. A detailed facilitator manual was developed to promote consistent delivery of the intervention across the UK.
Conclusions: We describe the development of an opioid reduction intervention package suitable for testing in the I-WOTCH randomised controlled trial.
Trial registration number: ISRCTN49470934.
Keywords: clinical trials; medical education & training; pain management; primary care; rehabilitation medicine.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: SE is the Chair of the specialised pain CRG at NHS England, he is Chief investigator and principal investigator of a number of NIHR and Industry funded trials, he has received personal fees from Medtronic Ltd, Mainstay Medical, Boston Scientific Corp for consultancy work. His department has received research funding from the National Institute of Health Research, Medtronic Ltd and Boston Scientific Corp. HS is director of Health Psychology Services Ltd, providing psychological services for a range of health related conditions. NKYT is chief investigator or coinvestigator of other chronic pain related projects funded by the NIHR, MRC, Warwick-Wellcome Translational Partnership. MU is chief investigator or coinvestigator on multiple previous and current research grants from the UK National Institute for Health Research, Arthritis Research UK and is a coinvestigator on grants funded by the Australian NHMRC. He was an NIHR Senior Investigator until March 2021. He has received travel expenses for speaking at conferences from the professional organisations hosting the conferences. He is a director and shareholder of Clinvivo Ltd that provides electronic data collection for health services research. He is part of an academic partnership with Serco Ltd, funded by the European Social Fund, related to return to work initiatives. He receives some salary support from University Hospitals Coventry and Warwickshire. He is a coinvestigator on three NIHR funded studies receiving additional support from Stryker Ltd. He has accepted honoraria for teaching/lecturing from consortium for advanced research training in Africa. Until March 2020, he was an editor of the NIHR journal series, and a member of the NIHR Journal Editors Group, for which he received a fee. ADF is author of the My Opioid Manager book and App distributed in iTunes and Google Play. Both book and app are free of charge. She is author of the Opioid Manager App, a paid app distributed only in iTunes for healthcare professionals. The app is owned by UHN, the hospital where ADF works. ADF does not get any financial benefit from the sales of the app. ADF has a monetized YouTube channel since January 2021 that contains some videos about opioids and opioid tapering. Since April 2021, ADF has an unrestricted educational grant to maintain an online self-assessment opioid course for healthcare professionals in Canada. The funding is provided by the Canadian Generics Pharmaceutical Association (CGPA). The funding organisation has no role in the preparation, approval, recruitment of participants, or data analysis of the course content. Responsibility for the course content is solely that of the authors. ST is chief investigator or coinvestigator on multiple previous and current research grants from the UK National Institute for Health Research.
Figures

References
-
- Vos T, Abajobir AA, Abate KH, et al. . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. The Lancet 2017;390:1211–59. 10.1016/S0140-6736(17)32154-2 - DOI - PMC - PubMed
-
- Bridges S. Health survey for England 2011, volume 1, health, social care and lifestyles. HSCIC, 2012: 291–306.
-
- International Association for the Study of Pain . Chronic Pain has arrived in the ICD-11 [Internet]. Washington: International Association for the study of Pain, 2019. Available: https://www.iasppain.org/PublicationsNews/NewsDetail.aspx?ItemNumber=8340
-
- Openprecribing, 2021. Available: https://openprescribing.net/analyse/#org=stp&numIds=4.7.2&denom=nothing&...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
