LncRNA GACAT2 binds with protein PKM1/2 to regulate cell mitochondrial function and cementogenesis in an inflammatory environment
- PMID: 35296649
- PMCID: PMC8927299
- DOI: 10.1038/s41413-022-00197-x
LncRNA GACAT2 binds with protein PKM1/2 to regulate cell mitochondrial function and cementogenesis in an inflammatory environment
Abstract
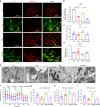
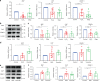
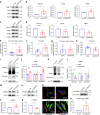
Periodontal ligament stem cells (PDLSCs) are a key cell type for restoring/regenerating lost/damaged periodontal tissues, including alveolar bone, periodontal ligament and root cementum, the latter of which is important for regaining tooth function. However, PDLSCs residing in an inflammatory environment generally exhibit compromised functions, as demonstrated by an impaired ability to differentiate into cementoblasts, which are responsible for regrowing the cementum. This study investigated the role of mitochondrial function and downstream long noncoding RNAs (lncRNAs) in regulating inflammation-induced changes in the cementogenesis of PDLSCs. We found that the inflammatory cytokine-induced impairment of the cementogenesis of PDLSCs was closely correlated with their mitochondrial function, and lncRNA microarray analysis and gain/loss-of-function studies identified GACAT2 as a regulator of the cellular events involved in inflammation-mediated mitochondrial function and cementogenesis. Subsequently, a comprehensive identification of RNA-binding proteins by mass spectrometry (ChIRP-MS) and parallel reaction monitoring (PRM) assays revealed that GACAT2 could directly bind to pyruvate kinase M1/2 (PKM1/2), a protein correlated with mitochondrial function. Further functional studies demonstrated that GACAT2 overexpression increased the cellular protein expression of PKM1/2, the PKM2 tetramer and phosphorylated PKM2, which led to enhanced pyruvate kinase (PK) activity and increased translocation of PKM2 into mitochondria. We then found that GACAT2 overexpression could reverse the damage to mitochondrial function and cementoblastic differentiation of PDLSCs induced by inflammation and that this effect could be abolished by PKM1/2 knockdown. Our data indicated that by binding to PKM1/2 proteins, the lncRNA GACAT2 plays a critical role in regulating mitochondrial function and cementogenesis in an inflammatory environment.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat. Rev. Dis. Prim. 2017;3:17038. - PubMed
-
- Bartold PM, Gronthos S, Ivanovski S, Fisher A, Hutmacher DW. Tissue engineered periodontal products. J. Periodontal Res. 2016;51:1–15. - PubMed
-
- Chen FM, Jin Y. Periodontal tissue engineering and regeneration: current approaches and expanding opportunities. Tissue Eng. Part B Rev. 2010;16:219–255. - PubMed
Grants and funding
- 81970947/National Natural Science Foundation of China (National Science Foundation of China)
- 81800971/National Natural Science Foundation of China (National Science Foundation of China)
- 82001102/National Natural Science Foundation of China (National Science Foundation of China)
- 2020JQ-447/Natural Science Foundation of Shanxi Province (Shanxi Province Natural Science Foundation)
- 2020JQ-444/Natural Science Foundation of Shandong Province (Shandong Provincial Natural Science Foundation)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

