Characterisation and induction of tissue-resident gamma delta T-cells to target hepatocellular carcinoma
- PMID: 35296658
- PMCID: PMC8927126
- DOI: 10.1038/s41467-022-29012-1
Characterisation and induction of tissue-resident gamma delta T-cells to target hepatocellular carcinoma
Abstract
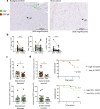
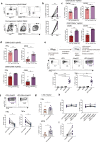
Immunotherapy is now the standard of care for advanced hepatocellular carcinoma (HCC), yet many patients fail to respond. A major unmet goal is the boosting of T-cells with both strong HCC reactivity and the protective advantages of tissue-resident memory T-cells (TRM). Here, we show that higher intratumoural frequencies of γδ T-cells, which have potential for HLA-unrestricted tumour reactivity, associate with enhanced HCC patient survival. We demonstrate that γδ T-cells exhibit bona fide tissue-residency in human liver and HCC, with γδTRM showing no egress from hepatic vasculature, persistence for >10 years and superior anti-tumour cytokine production. The Vγ9Vδ2 T-cell subset is selectively depleted in HCC but can efficiently target HCC cell lines sensitised to accumulate isopentenyl-pyrophosphate by the aminobisphosphonate Zoledronic acid. Aminobisphosphonate-based expansion of peripheral Vγ9Vδ2 T-cells recapitulates a TRM phenotype and boosts cytotoxic potential. Thus, our data suggest more universally effective HCC immunotherapy may be achieved by combining aminobisphosphonates to induce Vγ9Vδ2TRM capable of replenishing the depleted pool, with additional intratumoural delivery to sensitise HCC to Vγ9Vδ2TRM-based targeting.
© 2022. The Author(s).
Conflict of interest statement
Unrelated to the content of this manuscript, authors M.K.M. and N.M.S. have an international patent application No.1917498.6 entitled Treatment of Hepatitis B Virus (HBV) Infection filed by applicant UCL Business Ltd. MP is co-founder and director of Engitix Therapeutics Ltd, UK. The Maini lab has received unrestricted funding from Gilead, Roche and Immunocore. The remaining authors declare no competing interests.
Figures





References
-
- Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality Worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. - PubMed
-
- Finn RS, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020;382:1894–1905. - PubMed
-
- Llovet JM, et al. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021;7:6. - PubMed
-
- Prieto J, Melero I, Sangro B. Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2015;12:681–700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

