Tertiary lymphoid structures critical for prognosis in endometrial cancer patients
- PMID: 35296668
- PMCID: PMC8927106
- DOI: 10.1038/s41467-022-29040-x
Tertiary lymphoid structures critical for prognosis in endometrial cancer patients
Abstract
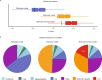
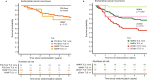
B-cells play a key role in cancer suppression, particularly when aggregated in tertiary lymphoid structures (TLS). Here, we investigate the role of B-cells and TLS in endometrial cancer (EC). Single cell RNA-sequencing of B-cells shows presence of naïve B-cells, cycling/germinal center B-cells and antibody-secreting cells. Differential gene expression analysis shows association of TLS with L1CAM overexpression. Immunohistochemistry and co-immunofluorescence show L1CAM expression in mature TLS, independent of L1CAM expression in the tumor. Using L1CAM as a marker, 378 of the 411 molecularly classified ECs from the PORTEC-3 biobank are evaluated, TLS are found in 19%. L1CAM expressing TLS are most common in mismatch-repair deficient (29/127, 23%) and polymerase-epsilon mutant EC (24/47, 51%). Multivariable Cox regression analysis shows strong favorable prognostic impact of TLS, independent of clinicopathological and molecular factors. Our data suggests a pivotal role of TLS in outcome of EC patients, and establishes L1CAM as a simple biomarker.
© 2022. The Author(s).
Conflict of interest statement
Dr. Horeweg reports outside of the submitted work to have received research grants from the Dutch Cancer Society (KWF-2021-13400, KWF-2021-13404). Dr. Church is funded by a Cancer Research UK Advanced Clinician Scientist Fellowship (C26642/A27963) and reports to be part of the advisory board for MSD. Prof. Nout, Dr. Bosse and Prof. Creutzberg report to have received a grant from the Dutch Cancer Society for the PORTEC-3 trial (KWF 2018-1-11629). Prof. Koelzer reports grants from Promedica Foundation (F-87701-41-01) during the conduct of the study and having served as an invited speaker on behalf of Indica Labs. Dr. de Bruyn reports, outside the submitted work, having received grants from the Dutch Cancer Society (KWF), grants from the European Research Council (ERC), grants from Health Holland, grants from DCPrime, non-financial support from BioNTech, non-financial support from Surflay, non-financial support from MSD, grants and non-financial support from Vicinivax. In addition, dr. de Bruyn has grants and non-financial support from Aduro Biotech, in part relating to a patent for Antibodies targeting CD103 (de Bruyn et al. No. 62/704,258). The remaining authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

