Corticosteroid sensitization drives opioid addiction
- PMID: 35296810
- PMCID: PMC10406162
- DOI: 10.1038/s41380-022-01501-1
Corticosteroid sensitization drives opioid addiction
Abstract
The global crisis of opioid overdose fatalities has led to an urgent search to discover the neurobiological mechanisms of opioid use disorder (OUD). A driving force for OUD is the dysphoric and emotionally painful state (hyperkatifeia) that is produced during acute and protracted opioid withdrawal. Here, we explored a mechanistic role for extrahypothalamic stress systems in driving opioid addiction. We found that glucocorticoid receptor (GR) antagonism with mifepristone reduced opioid addiction-like behaviors in rats and zebrafish of both sexes and decreased the firing of corticotropin-releasing factor neurons in the rat amygdala (i.e., a marker of brain stress system activation). In support of the hypothesized role of glucocorticoid transcriptional regulation of extrahypothalamic GRs in addiction-like behavior, an intra-amygdala infusion of an antisense oligonucleotide that blocked GR transcriptional activity reduced addiction-like behaviors. Finally, we identified transcriptional adaptations of GR signaling in the amygdala of humans with OUD. Thus, GRs, their coregulators, and downstream systems may represent viable therapeutic targets to treat the "stress side" of OUD.
© 2022. This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply.
Conflict of interest statement
Figures
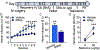

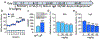



References
-
- Strang J, Volkow ND, Degenhardt L, Hickman M, Johnson K, Koob GF, et al. Opioid use disorder. Nat Rev Dis Primers. 2020;6:3. - PubMed
-
- Understanding the Epidemic | Drug Overdose | CDC Injury Center. 2019. https://www.cdc.gov/drugoverdose/epidemic/index.html. Accessed 7 January 2020.
-
- Koob GF. Neurobiology of Opioid Addiction: Opponent Process, Hyperkatifeia, and Negative Reinforcement. Biological Psychiatry. 2020;87:44–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

