Reproducible brain-wide association studies require thousands of individuals
- PMID: 35296861
- PMCID: PMC8991999
- DOI: 10.1038/s41586-022-04492-9
Reproducible brain-wide association studies require thousands of individuals
Erratum in
-
Publisher Correction: Reproducible brain-wide association studies require thousands of individuals.Nature. 2022 May;605(7911):E11. doi: 10.1038/s41586-022-04692-3. Nature. 2022. PMID: 35534626 Free PMC article. No abstract available.
Abstract
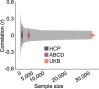
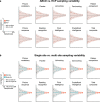
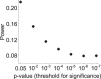
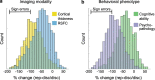
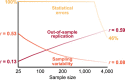
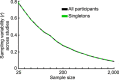
Magnetic resonance imaging (MRI) has transformed our understanding of the human brain through well-replicated mapping of abilities to specific structures (for example, lesion studies) and functions1-3 (for example, task functional MRI (fMRI)). Mental health research and care have yet to realize similar advances from MRI. A primary challenge has been replicating associations between inter-individual differences in brain structure or function and complex cognitive or mental health phenotypes (brain-wide association studies (BWAS)). Such BWAS have typically relied on sample sizes appropriate for classical brain mapping4 (the median neuroimaging study sample size is about 25), but potentially too small for capturing reproducible brain-behavioural phenotype associations5,6. Here we used three of the largest neuroimaging datasets currently available-with a total sample size of around 50,000 individuals-to quantify BWAS effect sizes and reproducibility as a function of sample size. BWAS associations were smaller than previously thought, resulting in statistically underpowered studies, inflated effect sizes and replication failures at typical sample sizes. As sample sizes grew into the thousands, replication rates began to improve and effect size inflation decreased. More robust BWAS effects were detected for functional MRI (versus structural), cognitive tests (versus mental health questionnaires) and multivariate methods (versus univariate). Smaller than expected brain-phenotype associations and variability across population subsamples can explain widespread BWAS replication failures. In contrast to non-BWAS approaches with larger effects (for example, lesions, interventions and within-person), BWAS reproducibility requires samples with thousands of individuals.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
E.A.E., D.A.F and N.U.F.D. have a financial interest in NOUS Imaging Inc. and may financially benefit if the company is successful in marketing FIRMM motion-monitoring software products. O.M.-D., E.A.E., A.N.V., D.A.F. and N.U.F.D. may receive royalty income based on FIRMM technology developed at Washington University School of Medicine and Oregon Health and Sciences University and licensed to NOUS Imaging Inc. D.A.F. and N.U.F.D. are co-founders of NOUS Imaging Inc. and E.A.E. is a former employee of NOUS Imaging. These potential conflicts of interest have been reviewed and are managed by Washington University School of Medicine, Oregon Health and Sciences University and the University of Minnesota. The other authors declare no competing interests.
Figures
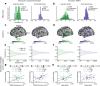
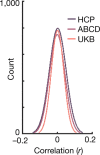
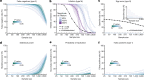
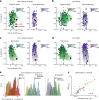
Comment in
-
Can brain scans reveal behaviour? Bombshell study says not yet.Nature. 2022 Mar;603(7903):777-778. doi: 10.1038/d41586-022-00767-3. Nature. 2022. PMID: 35301503 No abstract available.
-
Your brain expands and shrinks over time - these charts show how.Nature. 2022 Apr;604(7905):230-231. doi: 10.1038/d41586-022-00971-1. Nature. 2022. PMID: 35388158 No abstract available.
-
Brain-behavior correlations: Two paths toward reliability.Neuron. 2022 May 4;110(9):1446-1449. doi: 10.1016/j.neuron.2022.04.018. Neuron. 2022. PMID: 35512638
-
Beyond the AJR: Sampling Variability Impacts Reproducibility in Brain-Wide Association Studies.AJR Am J Roentgenol. 2023 Jan;220(1):149. doi: 10.2214/AJR.22.28026. Epub 2022 Jun 8. AJR Am J Roentgenol. 2023. PMID: 35674354 No abstract available.
-
How to establish robust brain-behavior relationships without thousands of individuals.Nat Neurosci. 2022 Jul;25(7):835-837. doi: 10.1038/s41593-022-01110-9. Nat Neurosci. 2022. PMID: 35710985 No abstract available.
-
The challenge of BWAs: Unknown unknowns in feature space and variance.Med. 2022 Aug 12;3(8):526-531. doi: 10.1016/j.medj.2022.07.002. Med. 2022. PMID: 35963233
-
Cognitive neuroscience at the crossroads.Nature. 2022 Aug;608(7924):647. doi: 10.1038/d41586-022-02283-w. Nature. 2022. PMID: 36002501 No abstract available.
-
Reply to: Multivariate BWAS can be replicable with moderate sample sizes.Nature. 2023 Mar;615(7951):E8-E12. doi: 10.1038/s41586-023-05746-w. Nature. 2023. PMID: 36890374 Free PMC article. No abstract available.
-
Multivariate BWAS can be replicable with moderate sample sizes.Nature. 2023 Mar;615(7951):E4-E7. doi: 10.1038/s41586-023-05745-x. Epub 2023 Mar 8. Nature. 2023. PMID: 36890392 Free PMC article. No abstract available.
References
MeSH terms
Grants and funding
- K12 DA043490/DA/NIDA NIH HHS/United States
- U24 DA041147/DA/NIDA NIH HHS/United States
- U01 DA041120/DA/NIDA NIH HHS/United States
- UE5 NS090978/NS/NINDS NIH HHS/United States
- R21 AA026919/AA/NIAAA NIH HHS/United States
- K23 NS123345/NS/NINDS NIH HHS/United States
- U24 DA041123/DA/NIDA NIH HHS/United States
- U01 DA041117/DA/NIDA NIH HHS/United States
- U01 DA041148/DA/NIDA NIH HHS/United States
- F31 NS110332/NS/NINDS NIH HHS/United States
- P50 HD103525/HD/NICHD NIH HHS/United States
- R25 NS090978/NS/NINDS NIH HHS/United States
- K23 NS088590/NS/NINDS NIH HHS/United States
- P50 NS022343/NS/NINDS NIH HHS/United States
- MC_QA137853/MRC_/Medical Research Council/United Kingdom
- U01 DA041093/DA/NIDA NIH HHS/United States
- U01 DA051038/DA/NIDA NIH HHS/United States
- T32 MH100019/MH/NIMH NIH HHS/United States
- U01 DA041134/DA/NIDA NIH HHS/United States
- U01 DA041022/DA/NIDA NIH HHS/United States
- K01 MH104592/MH/NIMH NIH HHS/United States
- K99 MH121518/MH/NIMH NIH HHS/United States
- U01 DA041156/DA/NIDA NIH HHS/United States
- U01 DA041025/DA/NIDA NIH HHS/United States
- R25 MH112473/MH/NIMH NIH HHS/United States
- K23 MH125023/MH/NIMH NIH HHS/United States
- R01 MH096773/MH/NIMH NIH HHS/United States
- U01 DA050989/DA/NIDA NIH HHS/United States
- U54 MH091657/MH/NIMH NIH HHS/United States
- U01 DA041089/DA/NIDA NIH HHS/United States
- U01 DA050988/DA/NIDA NIH HHS/United States
- U01 DA041106/DA/NIDA NIH HHS/United States
- T32 DA007261/DA/NIDA NIH HHS/United States
- U01 DA041028/DA/NIDA NIH HHS/United States
- U01 DA041048/DA/NIDA NIH HHS/United States
- T32 NS115672/NS/NINDS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- MC_PC_17228/MRC_/Medical Research Council/United Kingdom
- R44 MH121276/MH/NIMH NIH HHS/United States
- R01 MH115357/MH/NIMH NIH HHS/United States
- R44 MH122066/MH/NIMH NIH HHS/United States
- R44 MH124567/MH/NIMH NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- U01 DA041174/DA/NIDA NIH HHS/United States
- R00 MH121518/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

