Epicardial adipose tissue in contemporary cardiology
- PMID: 35296869
- PMCID: PMC8926097
- DOI: 10.1038/s41569-022-00679-9
Epicardial adipose tissue in contemporary cardiology
Abstract
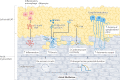
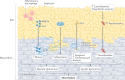
Interest in epicardial adipose tissue (EAT) is growing rapidly, and research in this area appeals to a broad, multidisciplinary audience. EAT is unique in its anatomy and unobstructed proximity to the heart and has a transcriptome and secretome very different from that of other fat depots. EAT has physiological and pathological properties that vary depending on its location. It can be highly protective for the adjacent myocardium through dynamic brown fat-like thermogenic function and harmful via paracrine or vasocrine secretion of pro-inflammatory and profibrotic cytokines. EAT is a modifiable risk factor that can be assessed with traditional and novel imaging techniques. Coronary and left atrial EAT are involved in the pathogenesis of coronary artery disease and atrial fibrillation, respectively, and it also contributes to the development and progression of heart failure. In addition, EAT might have a role in coronavirus disease 2019 (COVID-19)-related cardiac syndrome. EAT is a reliable potential therapeutic target for drugs with cardiovascular benefits such as glucagon-like peptide 1 receptor agonists and sodium-glucose co-transporter 2 inhibitors. This Review provides a comprehensive and up-to-date overview of the role of EAT in cardiovascular disease and highlights the translational nature of EAT research and its applications in contemporary cardiology.
© 2022. Springer Nature Limited.
Conflict of interest statement
The author declares no competing interests.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

