Anti-hypothalamus autoantibodies in anorexia nervosa: a possible new mechanism in neuro-physiological derangement?
- PMID: 35297008
- PMCID: PMC9556421
- DOI: 10.1007/s40519-022-01388-5
Anti-hypothalamus autoantibodies in anorexia nervosa: a possible new mechanism in neuro-physiological derangement?
Abstract
Purpose: Anorexia nervosa (AN) is a serious and complex mental disorder affecting mainly young adult women. AN patients are characterized by low body weight in combination with self-induced starvation, intense fear of gaining weight, and distortion of body image. AN is a multifactorial disease, linked by recent evidence to a dysregulation of the immune system.
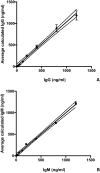
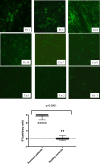
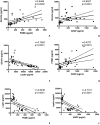
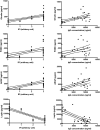
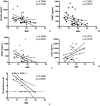
Methods: In this pilot study, 22 blood serums from AN patients were tested for the presence of autoantibodies against primate hypothalamic periventricular neurons by immunofluorescence and by a home-made ELISA assay. Cellular fluorescence suggests the presence of autoantibodies which are able to recognize these neurons (both to body cell and fiber levels). By means of ELISA, these autoantibodies are quantitatively evaluated. In addition, orexigenic and anorexigenic molecules were measured by ELISA. As control, 18 blood serums from healthy age matched woman were analysed.
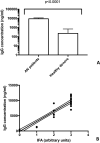
Results: All AN patients showed a reactivity against hypothalamic neurons both by immunofluorescence and ELISA. In addition, ghrelin, pro-opiomelanocortin (POMC), and agouti-related peptide (AGRP) were significantly higher than in control serums (p < 0.0001). In contrast, leptin was significantly lower in AN patients than controls (p < 0.0001).
Conclusions: Immunoreaction and ELISA assays on AN blood serum suggest the presence of autoantibodies AN related. However, it is not easy to determine the action of these antibodies in vivo: they could interact with specific ligands expressed by hypothalamic cells preventing their physiological role, however, it is also possible that they could induce an aspecific stimulation in the target cells leading to an increased secretion of anorexigenic molecules. Further studies are needed to fully understand the involvement of the immune system in AN pathogenesis.
Level of evidence: V, descriptive study.
Keywords: Anorexia nervosa; Anorexigenic molecules; Autoimmunity; Hypothalamic autoantibodies; Orexigenic molecules.
© 2022. The Author(s).
Conflict of interest statement
All the authors reported no medical financial interests or potential conflicts of interests.
Figures


Similar articles
-
Differential effects of methamphetamine on expression of neuropeptide Y mRNA in hypothalamus and on serum leptin and ghrelin concentrations in ad libitum-fed and schedule-fed rats.Neuroscience. 2005;132(1):167-73. doi: 10.1016/j.neuroscience.2004.11.037. Neuroscience. 2005. PMID: 15780475
-
Failure to upregulate Agrp and Orexin in response to activity based anorexia in weight loss vulnerable rats characterized by passive stress coping and prenatal stress experience.Psychoneuroendocrinology. 2016 May;67:171-81. doi: 10.1016/j.psyneuen.2016.02.002. Epub 2016 Feb 22. Psychoneuroendocrinology. 2016. PMID: 26907996 Free PMC article.
-
Ghrelin receptors mediate ghrelin-induced excitation of agouti-related protein/neuropeptide Y but not pro-opiomelanocortin neurons.J Neurochem. 2017 Aug;142(4):512-520. doi: 10.1111/jnc.14080. Epub 2017 Jun 21. J Neurochem. 2017. PMID: 28547758
-
The endocrine manifestations of anorexia nervosa: mechanisms and management.Nat Rev Endocrinol. 2017 Mar;13(3):174-186. doi: 10.1038/nrendo.2016.175. Epub 2016 Nov 4. Nat Rev Endocrinol. 2017. PMID: 27811940 Free PMC article. Review.
-
[Functional disturbances of the hypothalamus in patients with anorexia nervosa].Psychiatr Pol. 2004 Nov-Dec;38(6):1001-9. Psychiatr Pol. 2004. PMID: 15779664 Review. Polish.
Cited by
-
Dysfunction of Inflammatory Pathways and Their Relationship with Anti-Hypothalamic Autoantibodies in Patients with Anorexia Nervosa.Nutrients. 2023 May 5;15(9):2199. doi: 10.3390/nu15092199. Nutrients. 2023. PMID: 37432371 Free PMC article.
-
The Association between Blood SIRT1 and Ghrelin, Leptin, and Antibody Anti-Hypothalamus: A Comparison in Normal Weight and Anorexia Nervosa.J Pers Med. 2023 May 31;13(6):928. doi: 10.3390/jpm13060928. J Pers Med. 2023. PMID: 37373917 Free PMC article.
-
Serum kisspeptin and proopiomelanocortin in cystic fibrosis: a single study.Sci Rep. 2022 Oct 21;12(1):17669. doi: 10.1038/s41598-022-21851-8. Sci Rep. 2022. PMID: 36271282 Free PMC article.
-
Detection of natural autoimmunity to ghrelin in diabetes mellitus.Front Med Technol. 2024 Jul 12;6:1407409. doi: 10.3389/fmedt.2024.1407409. eCollection 2024. Front Med Technol. 2024. PMID: 39070294 Free PMC article.
-
Long-Term Course of Neural Autoantibody-Associated Psychiatric Disorders: Retrospective Data from a Specifically Immunopsychiatric Outpatient Clinic.Antibodies (Basel). 2023 May 8;12(2):34. doi: 10.3390/antib12020034. Antibodies (Basel). 2023. PMID: 37218900 Free PMC article.
References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

