Antiviral Activity of Olanexidine-Containing Hand Rub against Human Noroviruses
- PMID: 35297675
- PMCID: PMC9040745
- DOI: 10.1128/mbio.02848-21
Antiviral Activity of Olanexidine-Containing Hand Rub against Human Noroviruses
Abstract
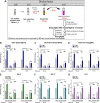
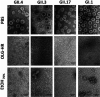
Human norovirus (HuNoV) is the leading cause of epidemic and sporadic acute gastroenteritis worldwide. HuNoV transmission occurs predominantly by direct person-to-person contact, and its health burden is associated with poor hand hygiene and a lack of effective antiseptics and disinfectants. Specific therapies and methods to prevent and control HuNoV spread previously were difficult to evaluate because of the lack of a cell culture system to propagate infectious virus. This barrier has been overcome with the successful cultivation of HuNoV in nontransformed human intestinal enteroids (HIEs). Here, we report using the HIE cultivation system to evaluate the virucidal efficacy of an olanexidine gluconate-based hand rub (OLG-HR) and 70% ethanol (EtOH70%) against HuNoVs. OLG-HR exhibited fast-acting virucidal activity against a spectrum of HuNoVs including GII.4 Sydney[P31], GII.4 Den Haag[P4], GII.4 New Orleans[P4], GII.3[P21], GII.17[P13], and GI.1[P1] strains. Exposure of HuNoV to OLG-HR for 30 to 60 s resulted in complete loss of the ability of virus to bind to the cells and reduced in vitro binding to glycans in porcine gastric mucin. By contrast, the virucidal efficiency of EtOH70% on virus infectivity was strain specific. Dynamic light scattering (DLS) and electron microscopy of virus-like particles (VLPs) show that OLG-HR treatment causes partial disassembly and possibly conformational changes in VP1, interfering with histo-blood group antigen (HBGA) binding and infectivity, whereas EtOH70% treatment causes particle disassembly and clumping of the disassembled products, leading to loss of infectivity while retaining HBGA binding. The highly effective inactivation of HuNoV infectivity by OLG-HR suggests that this compound could reduce HuNoV transmission. IMPORTANCE Human noroviruses (HuNoVs) are highly contagious and cause nonbacterial acute gastroenteritis in all age groups worldwide. Since the introduction of rotavirus vaccines, HuNoVs have become the leading cause of diarrheal illness in children. These viruses are very stable in the environment and resistant to common disinfectants. This study evaluated the virucidal efficacy of a new disinfectant, olanexidine-based hand rub (OLG-HR), against HuNoV strains in an ex vivo human intestinal stem cell-derived enteroid (HIE) cultivation system. Exposure of multiple HuNoV strains to OLG-HR for 30 to 60 s resulted in complete loss of infectivity and binding to HBGAs, possibly due to partial disassembly and conformational changes in the major virus capsid (VP1). By comparison, the virucidal efficiency of EtOH70% was strain specific, leading to loss of infectivity while retaining HBGA binding. These findings show the utility of the ex vivo HIE cultivation system to test the effectiveness of disinfectants and report a highly effective product.
Keywords: HIEs; HuNoV; IntestiCult media; OLG-HR; antiviral; noroviruses; olanexidine; organoids; virucidal activity.
Conflict of interest statement
The authors declare no conflict of interest. Baylor College of Medicine holds a patent on cultivation of human norovirus with Drs. Atmar, Estes, and Ettayebi as co-inventors.
Figures




Similar articles
-
Insights into human norovirus cultivation in human intestinal enteroids.mSphere. 2024 Nov 21;9(11):e0044824. doi: 10.1128/msphere.00448-24. Epub 2024 Oct 15. mSphere. 2024. PMID: 39404443 Free PMC article.
-
New Insights and Enhanced Human Norovirus Cultivation in Human Intestinal Enteroids.mSphere. 2021 Jan 27;6(1):e01136-20. doi: 10.1128/mSphere.01136-20. mSphere. 2021. PMID: 33504663 Free PMC article.
-
Insights into Human Norovirus Cultivation in Human Intestinal Enteroids.bioRxiv [Preprint]. 2024 Sep 19:2024.05.24.595764. doi: 10.1101/2024.05.24.595764. bioRxiv. 2024. Update in: mSphere. 2024 Nov 21;9(11):e0044824. doi: 10.1128/msphere.00448-24. PMID: 38826387 Free PMC article. Updated. Preprint.
-
Interaction between norovirus and Histo-Blood Group Antigens: A key to understanding virus transmission and inactivation through treatments?Food Microbiol. 2020 Dec;92:103594. doi: 10.1016/j.fm.2020.103594. Epub 2020 Jul 14. Food Microbiol. 2020. PMID: 32950136 Review.
-
Glycan Recognition in Human Norovirus Infections.Viruses. 2021 Oct 14;13(10):2066. doi: 10.3390/v13102066. Viruses. 2021. PMID: 34696500 Free PMC article. Review.
Cited by
-
Insights into human norovirus cultivation in human intestinal enteroids.mSphere. 2024 Nov 21;9(11):e0044824. doi: 10.1128/msphere.00448-24. Epub 2024 Oct 15. mSphere. 2024. PMID: 39404443 Free PMC article.
-
Standardization of an antiviral pipeline for human norovirus in human intestinal enteroids demonstrates nitazoxanide has no to weak antiviral activity.Antimicrob Agents Chemother. 2023 Oct 18;67(10):e0063623. doi: 10.1128/aac.00636-23. Epub 2023 Oct 3. Antimicrob Agents Chemother. 2023. PMID: 37787556 Free PMC article.
-
CLIC and membrane wound repair pathways enable pandemic norovirus entry and infection.Nat Commun. 2023 Feb 28;14(1):1148. doi: 10.1038/s41467-023-36398-z. Nat Commun. 2023. PMID: 36854760 Free PMC article.
-
A Standardized Antiviral Pipeline for Human Norovirus in Human Intestinal Enteroids Demonstrates No Antiviral Activity of Nitazoxanide.bioRxiv [Preprint]. 2023 May 23:2023.05.23.542011. doi: 10.1101/2023.05.23.542011. bioRxiv. 2023. Update in: Antimicrob Agents Chemother. 2023 Oct 18;67(10):e0063623. doi: 10.1128/aac.00636-23. PMID: 37293103 Free PMC article. Updated. Preprint.
-
Applications of human organoids in the personalized treatment for digestive diseases.Signal Transduct Target Ther. 2022 Sep 27;7(1):336. doi: 10.1038/s41392-022-01194-6. Signal Transduct Target Ther. 2022. PMID: 36167824 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

