Effectiveness, Cost-effectiveness, and Cost-Utility of a Digital Smoking Cessation Intervention for Cancer Survivors: Health Economic Evaluation and Outcomes of a Pragmatic Randomized Controlled Trial
- PMID: 35297777
- PMCID: PMC9491833
- DOI: 10.2196/27588
Effectiveness, Cost-effectiveness, and Cost-Utility of a Digital Smoking Cessation Intervention for Cancer Survivors: Health Economic Evaluation and Outcomes of a Pragmatic Randomized Controlled Trial
Abstract
Background: Smoking cessation (SC) interventions may contribute to better treatment outcomes and the general well-being of cancer survivors.
Objective: This study aims to evaluate the effectiveness, cost-effectiveness, and cost-utility of a digital interactive SC intervention compared with a noninteractive web-based information brochure for cancer survivors.
Methods: A health economic evaluation alongside a pragmatic 2-arm parallel-group randomized controlled trial was conducted with follow-ups at 3, 6, and 12 months. The study was conducted in the Netherlands over the internet from November 2016 to September 2019. The participants were Dutch adult smoking cancer survivors with the intention to quit smoking. In total, 165 participants were included and analyzed: 83 (50.3%) in the MyCourse group and 82 (49.7%) in the control group. In the intervention group, participants had access to a newly developed, digital, minimally guided SC intervention (MyCourse-Quit Smoking). Control group participants received a noninteractive web-based information brochure on SC. Both groups received unrestricted access to usual care. The primary outcome was self-reported 7-day smoking abstinence at the 6-month follow-up. Secondary outcomes were quality-adjusted life years gained, number of cigarettes smoked, nicotine dependence, and treatment satisfaction. For the health economic evaluation, intervention costs, health care costs, and costs stemming from productivity losses were assessed over a 12-month horizon.
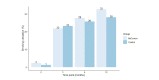
Results: At the 6-month follow-up, the quit rates were 28% (23/83) and 26% (21/82) in the MyCourse and control groups, respectively (odds ratio 0.47, 95% CI 0.03-7.86; P=.60). In both groups, nicotine dependence scores were reduced at 12 months, and the number of smoked cigarettes was reduced by approximately half. The number of cigarettes decreased more over time, and the MyCourse group demonstrated a significantly greater reduction at the 12-month follow-up (incidence rate ratio 0.87; 95% CI 0.76-1.00; P=.04). Intervention costs were estimated at US $193 per participant for the MyCourse group and US $74 for the control group. The mean per-participant societal costs were US $25,329 (SD US $29,137) and US $21,836 (SD US $25,792), respectively. In the cost-utility analysis, MyCourse was not preferred over the control group from a societal perspective. With smoking behavior as the outcome, the MyCourse group led to marginally better results per reduced pack-year against higher societal costs, with a mean incremental cost-effectiveness ratio of US $52,067 (95% CI US $32,515-US $81,346).
Conclusions: At 6 months, there was no evidence of a differential effect on cessation rates; in both groups, approximately a quarter of the cancer survivors quit smoking and their number of cigarettes smoked was reduced by half. At 12 months, the MyCourse intervention led to a greater reduction in the number of smoked cigarettes, albeit at higher costs than for the control group. No evidence was found for a differential effect on quality-adjusted life years.
Trial registration: The Netherlands Trial Register NTR6011; https://www.trialregister.nl/trial/5434.
International registered report identifier (irrid): RR2-10.1186/s12885-018-4206-z.
Keywords: cancer survivors; cost-effectiveness; eHealth; effectiveness; smoking cessation.
©Ajla Mujcic, Matthijs Blankers, Brigitte Boon, Irma M Verdonck-de Leeuw, Filip Smit, Margriet van Laar, Rutger Engels. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 17.03.2022.
Conflict of interest statement
Conflicts of Interest: The intervention described in this study was developed by the Trimbos Institute (the Netherlands Institute for Mental Health and Addiction).
Figures


Similar articles
-
Digital Smoking Cessation Intervention for Cancer Survivors: Analysis of Predictors and Moderators of Engagement and Outcome Alongside a Randomized Controlled Trial.JMIR Cancer. 2024 Jun 20;10:e46303. doi: 10.2196/46303. JMIR Cancer. 2024. PMID: 38901028 Free PMC article.
-
Effectiveness, Cost-effectiveness, and Cost-Utility of a Digital Alcohol Moderation Intervention for Cancer Survivors: Health Economic Evaluation and Outcomes of a Pragmatic Randomized Controlled Trial.J Med Internet Res. 2022 Feb 1;24(2):e30095. doi: 10.2196/30095. J Med Internet Res. 2022. PMID: 35103605 Free PMC article. Clinical Trial.
-
Internet-based self-help smoking cessation and alcohol moderation interventions for cancer survivors: a study protocol of two RCTs.BMC Cancer. 2018 Apr 2;18(1):364. doi: 10.1186/s12885-018-4206-z. BMC Cancer. 2018. PMID: 29609554 Free PMC article.
-
The effectiveness of distance-based interventions for smoking cessation and alcohol moderation among cancer survivors: A meta-analysis.Psychooncology. 2020 Jan;29(1):49-60. doi: 10.1002/pon.5261. Epub 2019 Dec 21. Psychooncology. 2020. PMID: 31663182 Free PMC article. Review. German.
-
Cluster randomized controlled trial of a patient and general practitioner intervention to improve the management of multiple risk factors after stroke: stop stroke.Stroke. 2010 Nov;41(11):2470-6. doi: 10.1161/STROKEAHA.110.588046. Epub 2010 Sep 23. Stroke. 2010. PMID: 20864664 Review.
Cited by
-
Digital Smoking Cessation Intervention for Cancer Survivors: Analysis of Predictors and Moderators of Engagement and Outcome Alongside a Randomized Controlled Trial.JMIR Cancer. 2024 Jun 20;10:e46303. doi: 10.2196/46303. JMIR Cancer. 2024. PMID: 38901028 Free PMC article.
-
Technology-based interventions for tobacco smoking prevention and treatment: a 20-year bibliometric analysis (2003-2022).Subst Abuse Treat Prev Policy. 2024 Feb 6;19(1):13. doi: 10.1186/s13011-024-00595-w. Subst Abuse Treat Prev Policy. 2024. PMID: 38321493 Free PMC article.
-
Cause of Death in Patients with Oropharyngeal Carcinoma by Human Papillomavirus Status: Comparative Data Analysis.JMIR Public Health Surveill. 2023 Aug 29;9:e47579. doi: 10.2196/47579. JMIR Public Health Surveill. 2023. PMID: 37642982 Free PMC article.
-
eHealth interventions for psychiatry in Switzerland and Russia: a comparative study.Front Digit Health. 2024 Jan 19;5:1278176. doi: 10.3389/fdgth.2023.1278176. eCollection 2023. Front Digit Health. 2024. PMID: 38314194 Free PMC article.
References
-
- National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health . The health consequences of smoking—50 years of progress: a report of the surgeon general. Atlanta, GA: Centers for Disease Control and Prevention; 2014. pp. 7–13. - PubMed
-
- Cancer trends progress report: cancer survivors and smoking. National Cancers Institute. 2020. [2020-05-15]. https://web.archive.org/web/20200515122922/https://progressreport.cancer... .
-
- Gallaway MS, Glover-Kudon R, Momin B, Puckett M, Lunsford NB, Ragan KR, Rohan EA, Babb S. Smoking cessation attitudes and practices among cancer survivors - United States, 2015. J Cancer Surviv. 2019;13(1):66–74. doi: 10.1007/s11764-018-0728-2. http://europepmc.org/abstract/MED/30612253 10.1007/s11764-018-0728-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

