Craving of prescription opioids among veterans with chronic pain
- PMID: 35297818
- PMCID: PMC9329486
- DOI: 10.1097/j.pain.0000000000002598
Craving of prescription opioids among veterans with chronic pain
Abstract
The United States faces a crisis because of the high prevalence of chronic pain, concurrent opioid use disorder, and overdose deaths. Prescription opioids remain a primary driver of opioid-related deaths. Craving is a core symptom of addiction, yet the degree to which craving plays a role in prescription opioid use among patients with chronic pain is unknown. Understanding the degree to which craving should be considered in patients with chronic pain is critical for developing effective interventions for supporting patients through opioid tapering. The current work combines data collected from (1) 2152 veterans screened for eligibility at a pain specialty care clinic at the San Francisco VA Health Care System and (2) medical records obtained from the VA Corporate Data Warehouse. We found that prescription opioid craving among veterans with chronic pain was low, with 66.4% of the sample reporting no craving and 33.6% reporting craving. We also found that craving had a small association with morphine equivalent daily dose and pain severity but was more strongly associated with depression. Craving of prescription opioids among veterans with chronic pain is complex. Findings are discussed in relation to chronic pain symptoms, psychiatric comorbidities, and demographics.
Copyright © 2022 International Association for the Study of Pain.
Conflict of interest statement
The authors present no conflicts of interest.
Figures

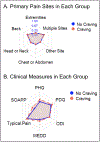

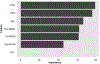
References
-
- Anagnostis C, Gatchel RJ, Mayer TG. The Pain Disability Questionnaire: A New Psychometrically Sound Measure for Chronic Musculoskeletal Disorders. Spine 2004;29(20):2290–2302 2210.1097/2201.brs.0000142221.0000188111.0000142220f. - PubMed
-
- Anton RF, Moak DH, Latham PK. The obsessive compulsive drinking scale: A new method of assessing outcome in alcoholism treatment studies. Arch Gen Psychiatry 1996;53(3):225–231. - PubMed
-
- Association AP. Diagnostic and statistical manual of mental disorders (DSM-5®): American Psychiatric Pub, 2013. - PubMed

