Synaptic pruning of murine adult-born neurons by microglia depends on phosphatidylserine
- PMID: 35297954
- PMCID: PMC9195048
- DOI: 10.1084/jem.20202304
Synaptic pruning of murine adult-born neurons by microglia depends on phosphatidylserine
Abstract
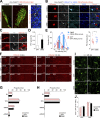
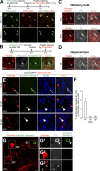
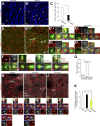
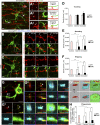
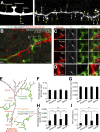
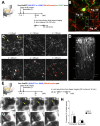
New neurons, continuously added in the adult olfactory bulb (OB) and hippocampus, are involved in information processing in neural circuits. Here, we show that synaptic pruning of adult-born neurons by microglia depends on phosphatidylserine (PS), whose exposure on dendritic spines is inversely correlated with their input activity. To study the role of PS in spine pruning by microglia in vivo, we developed an inducible transgenic mouse line, in which the exposed PS is masked by a dominant-negative form of milk fat globule-EGF-factor 8 (MFG-E8), MFG-E8D89E. In this transgenic mouse, the spine pruning of adult-born neurons by microglia is impaired in the OB and hippocampus. Furthermore, the electrophysiological properties of these adult-born neurons are altered in MFG-E8D89E mice. These data suggest that PS is involved in the microglial spine pruning and the functional maturation of adult-born neurons. The MFG-E8D89E-based genetic approach shown in this study has broad applications for understanding the biology of PS-mediated phagocytosis in vivo.
© 2022 Kurematsu et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures








References
-
- Breton-Provencher, V., Cote D., and Saghatelyan A.. 2014. Activity of the principal cells of the olfactory bulb promotes a structural dynamic on the distal dendrites of immature adult-born granule cells via activation of NMDA receptors. J. Neurosci. 34:1748–1759. 10.1523/JNEUROSCI.3013-13.2014 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

