The Blimp-1 transcription factor acts in non-neuronal cells to regulate terminal differentiation of the Drosophila eye
- PMID: 35297965
- PMCID: PMC8995086
- DOI: 10.1242/dev.200217
The Blimp-1 transcription factor acts in non-neuronal cells to regulate terminal differentiation of the Drosophila eye
Abstract
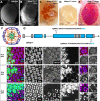
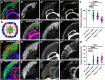
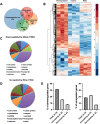
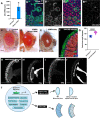
The formation of a functional organ such as the eye requires specification of the correct cell types and their terminal differentiation into cells with the appropriate morphologies and functions. Here, we show that the zinc-finger transcription factor Blimp-1 acts in secondary and tertiary pigment cells in the Drosophila retina to promote the formation of a bi-convex corneal lens with normal refractive power, and in cone cells to enable complete extension of the photoreceptor rhabdomeres. Blimp-1 expression depends on the hormone ecdysone, and loss of ecdysone signaling causes similar differentiation defects. Timely termination of Blimp-1 expression is also important, as its overexpression in the eye has deleterious effects. Our transcriptomic analysis revealed that Blimp-1 regulates the expression of many structural and secreted proteins in the retina. Blimp-1 may function in part by repressing another transcription factor; Slow border cells is highly upregulated in the absence of Blimp-1, and its overexpression reproduces many of the effects of removing Blimp-1. This work provides insight into the transcriptional networks and cellular interactions that produce the structures necessary for visual function.
Keywords: Drosophila; Blimp-1; Cone cells; Corneal lens; Pigment cells; Rhabdomeres.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


References
-
- Agawa, Y., Sarhan, M., Kageyama, Y., Akagi, K., Takai, M., Hashiyama, K., Wada, T., Handa, H., Iwamatsu, A., Hirose, S.et al. (2007). Drosophila Blimp-1 is a transient transcriptional repressor that controls timing of the ecdysone-induced developmental pathway. Mol. Cell. Biol. 27, 8739-8747. 10.1128/MCB.01304-07 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

