Healthcare resource utilization trends in patients with acute myeloid leukemia ineligible for intensive chemotherapy receiving first-line systemic treatment or best supportive care: A multicenter international study
- PMID: 35298049
- PMCID: PMC9324937
- DOI: 10.1111/ejh.13769
Healthcare resource utilization trends in patients with acute myeloid leukemia ineligible for intensive chemotherapy receiving first-line systemic treatment or best supportive care: A multicenter international study
Abstract
Objectives: This retrospective chart review examined real-world healthcare resource utilization (HRU) in patients with AML ineligible for intensive therapy who received first-line systemic therapy or best supportive care (BSC).
Methods: Data were collected anonymously on patients with AML who initiated first-line hypomethylating agents (HMA), low-dose cytarabine (LDAC), other systemic therapy, or BSC. HRU endpoints included hospitalizations, outpatient consultations, transfusions, and supportive care.
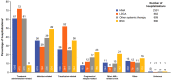
Results: Of 1762 patients included, 46% received HMA, 11% received LDAC, 17% received other systemic therapy, 26% received BSC; median treatment durations were 118, 35, 33, and 57 days, respectively. Most patients were hospitalized, most commonly for treatment administration, transfusion, or infection (HMA 82%, LDAC 93%, other systemic therapy 83%, BSC 83%). A median number of hospitalizations were 2-6 across systemic groups and two for BSC, with median durations of 8-18 days. Transfusion rates and outpatient consultations were highest for HMA (80% and 79%) versus LDAC (57% and 53%), other systemic therapy (57% and 63%), and BSC (71% and 66%). Antivirals/antibiotics and antifungals were used more frequently than growth factors (72-92%, 34-63%, and 7-27%, respectively).
Conclusion: Patients with AML ineligible for intensive therapy have high HRU; novel therapies are needed to alleviate this burden.
Keywords: AML; best supportive care; healthcare resource utilization; hypomethylating agents; low-dose cytarabine; low-intensity therapy.
© 2022 The Authors. European Journal of Haematology published by John Wiley & Sons Ltd.
Conflict of interest statement
T. Ito: Advisory role for AbbVie and speaker honoraria for AbbVie, BMS, Novartis, Sanofi, Takeda. D. Sanford: Advisory role for AbbVie, Astellas, Novartis, Pfizer. C. Tomuleasa: No potential conflicts of interest are reported. H.‐H. Hsiao: Advisory role for AbbVie, Amgen, Janssen, Novartis, Pfizer. L. J. Enciso Olivera: No potential conflicts of interest are reported. A. K. Enjeti: Advisory role for AbbVie, Astellas, Novartis, Alexion, and Jazz Pharmaceuticals. Speaker for Alexion, Bayer, Sanofi. A. Gimenez Conca: No potential conflicts of interest are reported. T. Bernal del Castillo: No potential conflicts of interest are reported. L. Girshova: No potential conflicts of interest are reported. M. P. Martelli: Advisory role for AbbVie, Amgen, Celgene, Janssen, Jazz Pharmaceuticals, Novartis, Pfizer. Speaker honoraria for Amgen, Celgene, Janssen, Novartis. B. Guvenc: Advisory role for AbbVie. C. Bui, A. Delgado, Y. Duan, B. Garbayo Guijarro, C. Llamas: Employees of AbbVie and may hold stock or options. J.‐H. Lee: Advisory role for AbbVie, Astellas, Celgene, Janssen, Novartis.
Figures

Similar articles
-
Real-world treatment patterns and clinical outcomes in patients with AML unfit for first-line intensive chemotherapy.Leuk Lymphoma. 2022 Apr;63(4):928-938. doi: 10.1080/10428194.2021.2002321. Epub 2022 Feb 11. Leuk Lymphoma. 2022. PMID: 35147482
-
Real-world treatment patterns and clinical outcomes in patients with AML in Japan who were ineligible for first-line intensive chemotherapy.Int J Hematol. 2022 Jul;116(1):89-101. doi: 10.1007/s12185-022-03334-8. Epub 2022 Apr 8. Int J Hematol. 2022. PMID: 35394258
-
Treatment regimens in patients over 64 years with acute myeloid leukaemia: a retrospective single-institution, multi-site analysis.Hematology. 2023 Dec;28(1):2206694. doi: 10.1080/16078454.2023.2206694. Epub 2023 May 11. Hematology. 2023. PMID: 38078486
-
Selection and management of older patients with acute myeloid leukemia treated with glasdegib plus low-dose cytarabine: expert panel review.Leuk Lymphoma. 2020 Dec;61(14):3287-3305. doi: 10.1080/10428194.2020.1817445. Epub 2020 Sep 24. Leuk Lymphoma. 2020. PMID: 32967493 Review.
-
An evaluation of venetoclax in combination with azacitidine, decitabine, or low-dose cytarabine as therapy for acute myeloid leukemia.Expert Rev Hematol. 2021 May;14(5):407-417. doi: 10.1080/17474086.2021.1938533. Epub 2021 Jun 15. Expert Rev Hematol. 2021. PMID: 34076549 Free PMC article. Review.
Cited by
-
Real-World Treatment Patterns and Clinical Outcomes in Canadian Patients with AML Unfit for First-Line Intensive Chemotherapy.Curr Oncol. 2022 Sep 22;29(10):6794-6806. doi: 10.3390/curroncol29100535. Curr Oncol. 2022. PMID: 36290812 Free PMC article.
-
2-Hydroxyglutarate in Acute Myeloid Leukemia: A Journey from Pathogenesis to Therapies.Biomedicines. 2022 Jun 9;10(6):1359. doi: 10.3390/biomedicines10061359. Biomedicines. 2022. PMID: 35740380 Free PMC article. Review.
-
Real-world treatments and clinical outcomes in unfit AML patients receiving first-line treatment or best supportive care in Italy (CURRENT study).Leuk Res Rep. 2024 Feb 23;21:100453. doi: 10.1016/j.lrr.2024.100453. eCollection 2024. Leuk Res Rep. 2024. PMID: 39035747 Free PMC article.
-
Modification of the Antibiotic, Colistin, with Dextrin Causes Enhanced Cytotoxicity and Triggers Apoptosis in Myeloid Leukemia.Int J Nanomedicine. 2024 Jun 7;19:5419-5437. doi: 10.2147/IJN.S449185. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38868592 Free PMC article.
References
-
- Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373:1136‐1152. - PubMed
-
- Institute NC . Cancer Stat Facts: Leukemia ‐ Acute Myeloid Leukemia. Accessed February 5, 2021. https://seer.cancer.gov/statfacts/html/amyl.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

