Relation of sensorimotor and cognitive cerebellum functional connectivity with brain structural damage in patients with multiple sclerosis and no disability
- PMID: 35298059
- PMCID: PMC9323479
- DOI: 10.1111/ene.15329
Relation of sensorimotor and cognitive cerebellum functional connectivity with brain structural damage in patients with multiple sclerosis and no disability
Abstract
Background and purpose: To investigate the relationship between the functional connectivity (FC) of the sensorimotor and cognitive cerebellum and measures of structural damage in patients with multiple sclerosis (MS) and no physical disability.
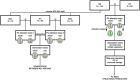
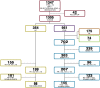
Methods: We selected 144 relapsing-remitting MS patients with an Expanded Disability Status Scale score of ≤1.5 and 98 healthy controls from the Italian Neuroimaging Network Initiative database. From multimodal 3T magnetic resonance imaging (MRI), including functional MRI at rest, we calculated lesion load, cortical thickness, and white matter, cortical gray matter, and caudate, putamen, thalamic, and cerebellar volumes. Voxel-wise FC of the sensorimotor and cognitive cerebellum was assessed with seed-based analysis, and multiple regression analysis was used to evaluate the relationship between FC and structural damage.
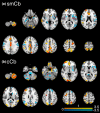
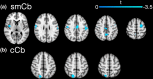
Results: Whole brain, white matter, caudate, putamen, and thalamic volumes were reduced in patients compared to controls, whereas cortical gray matter was not significantly different in patients versus controls. Both the sensorimotor and cognitive cerebellum showed a widespread pattern of increased and decreased FC that were negatively associated with structural measures, indicating that the lower the FC, the greater the tissue loss. Lastly, among multiple structural measures, cortical gray matter and white matter volumes were the best predictors of cerebellar FC alterations.
Conclusions: Increased and decreased cerebellar FC with several brain areas coexist in MS patients with no disability. Our data suggest that white matter loss hampers FC, whereas, in the absence of atrophy, cortical volume represents the framework for FC to increase.
Keywords: cognitive cerebellum; disability; functional magnetic resonance imaging; multiples sclerosis; neural plasticity; sensorimotor cerebellum.
© 2022 The Authors. European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
S.T., V.I. and C.G. have nothing to disclose. M.A.R. received speaker honoraria from Bayer, Biogen Idec, Celgene, Genzyme, Merck Serono, Novartis, Roche, and Teva, and receives research support from the MS Society of Canada and Fondazione Italiana Sclerosi Multipla. N.P. received speaker fees from Biogen and mission support from Genzyme and Novartis. G.T. received funding for travel, speaking honorarium, consulting service, and research projects from Novartis, Genzyme, Teva, Merck, Biogen, Roche, Allergan, Abbvie, Lilly, and Mylan. N.D.S. has received honoraria from Biogen‐Idec, Celgene, Immunic, Merck Serono, Novartis, Roche, Sanofi‐Genzyme, and Teva for consulting services, speaking, and travel support. He serves on advisory boards for Biogen‐Idec, Immunic, Merck Serono, Novartis, Roche, and Sanofi‐Genzyme. He has received research grant support from the Italian Multiple Sclerosis Society. C.P. received consulting and lecture fees from Sanofi‐Aventis, Biogen Idec, Bayer Schering, Merck Serono, and Novartis. He also received research funding from Novartis, Sanofi‐Aventis, Merck Serono, and Bayer Schering. M.F. is Editor‐in‐Chief of the
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

