Lipid Phase Separation in Vesicles Enhances TRAIL-Mediated Cytotoxicity
- PMID: 35298184
- PMCID: PMC9680886
- DOI: 10.1021/acs.nanolett.1c04365
Lipid Phase Separation in Vesicles Enhances TRAIL-Mediated Cytotoxicity
Abstract
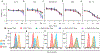
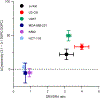
Ligand spatial presentation and density play important roles in signaling pathways mediated by cell receptors and are critical parameters when designing protein-conjugated therapeutic nanoparticles. Here, we harness lipid phase separation to spatially control the protein presentation on lipid vesicles. We use this system to improve the cytotoxicity of TNF-related apoptosis inducing ligand (TRAIL), a therapeutic anticancer protein. Vesicles with phase-separated TRAIL presentation induce more cell death in Jurkat cancer cells than vesicles with uniformly presented TRAIL, and cytotoxicity is dependent on TRAIL density. We assess this relationship in other cancer cell lines and demonstrate that phase-separated vesicles with TRAIL only enhance cytotoxicity through one TRAIL receptor, DR5, while another TRAIL receptor, DR4, is less sensitive to TRAIL density. This work demonstrates a rapid and accessible method to control protein conjugation and density on vesicles that can be adopted to other nanoparticle systems to improve receptor signaling by nanoparticles.
Keywords: Janus particles; TRAIL; lipid domains; liposomes; phase separation; vesicles.
Conflict of interest statement
The authors declare the following competing financial interest(s): N.P.K, J.A.P, and T.Q.V. are inventors on a provisional patent filing related to the technology described here.
Figures



References
-
- Ye K; Wang X; Cao L; Li S; Li Z; Yu L; Ding J Matrix Stiffness and Nanoscale Spatial Organization of Cell-Adhesive Ligands Direct Stem Cell Fate. Nano Lett. 2015, 15, 4720–4729. - PubMed
-
- Smith MR; Tolbert SV; Wen F Protein-Scaffold Directed Nanoscale Assembly of T Cell Ligands: Artificial Antigen Presentation with Defined Valency, Density, and Ratio. ACS Synth. Biol 2018, 7, 1629–1639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

