Imaging Modality and Frequency in Surveillance of Stage I Seminoma Testicular Cancer: Results From a Randomized, Phase III, Noninferiority Trial (TRISST)
- PMID: 35298280
- PMCID: PMC7614664
- DOI: 10.1200/JCO.21.01199
Imaging Modality and Frequency in Surveillance of Stage I Seminoma Testicular Cancer: Results From a Randomized, Phase III, Noninferiority Trial (TRISST)
Abstract
Purpose: Survival in stage I seminoma is almost 100%. Computed tomography (CT) surveillance is an international standard of care, avoiding adjuvant therapy. In this young population, minimizing irradiation is vital. The Trial of Imaging and Surveillance in Seminoma Testis (TRISST) assessed whether magnetic resonance images (MRIs) or a reduced scan schedule could be used without an unacceptable increase in advanced relapses.
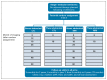
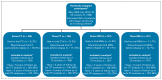
Methods: A phase III, noninferiority, factorial trial. Eligible participants had undergone orchiectomy for stage I seminoma with no adjuvant therapy planned. Random assignment was to seven CTs (6, 12, 18, 24, 36, 48, and 60 months); seven MRIs (same schedule); three CTs (6, 18, and 36 months); or three MRIs. The primary outcome was 6-year incidence of Royal Marsden Hospital stage ≥ IIC relapse (> 5 cm), aiming to exclude increases ≥ 5.7% (from 5.7% to 11.4%) with MRI (v CT) or three scans (v 7); target N = 660, all contributing to both comparisons. Secondary outcomes include relapse ≥ 3 cm, disease-free survival, and overall survival. Intention-to-treat and per-protocol analyses were performed.
Results: Six hundred sixty-nine patients enrolled (35 UK centers, 2008-2014); mean tumor size was 2.9 cm, and 358 (54%) were low risk (< 4 cm, no rete testis invasion). With a median follow-up of 72 months, 82 (12%) relapsed. Stage ≥ IIC relapse was rare (10 events). Although statistically noninferior, more events occurred with three scans (nine, 2.8%) versus seven scans (one, 0.3%): 2.5% absolute increase, 90% CI (1.0 to 4.1). Only 4/9 could have potentially been detected earlier with seven scans. Noninferiority of MRI versus CT was also shown; fewer events occurred with MRI (two [0.6%] v eight [2.6%]), 1.9% decrease (-3.5 to -0.3). Per-protocol analyses confirmed noninferiority. Five-year survival was 99%, with no tumor-related deaths.
Conclusion: Surveillance is a safe management approach-advanced relapse is rare, salvage treatment successful, and outcomes excellent, regardless of imaging frequency or modality. MRI can be recommended to reduce irradiation; and no adverse impact on long-term outcomes was seen with a reduced schedule.
Trial registration: ClinicalTrials.gov NCT00589537.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures
References
-
- Bray F, Ferlay J, Devesa SS, et al. Interpreting the international trends in testicular seminoma and nonseminoma incidence. Nat Clin Pract Urol. 2006;3:532–543. - PubMed
-
- Tandstad T, Smaaland R, Solberg A, et al. Management of seminomatous testicular cancer: A binational prospective population-based study from the Swedish Norwegian testicular cancer study group. J Clin Oncol. 2011;29:719–725. - PubMed
-
- Cafferty FH, Gabe R, Huddart RA, et al. UK management practices in stage I seminoma and the Medical Research Council Trial of Imaging and Schedule in Seminoma Testis managed with surveillance. Clin Oncol (R Coll Radiol) 2012;24:25–29. - PubMed
-
- Dieckmann KP, Dralle-Filiz I, Heinzelbecker J, et al. Seminoma clinical stage 1-patterns of care in Germany. Urol Int. 2016;96:390–398. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

