Phase II Trial of Cabozantinib Plus Nivolumab in Patients With Non-Clear-Cell Renal Cell Carcinoma and Genomic Correlates
- PMID: 35298296
- PMCID: PMC9287282
- DOI: 10.1200/JCO.21.01944
Phase II Trial of Cabozantinib Plus Nivolumab in Patients With Non-Clear-Cell Renal Cell Carcinoma and Genomic Correlates
Abstract
Purpose: To assess the efficacy and safety of cabozantinib plus nivolumab in a phase II trial in patients with non-clear-cell renal cell carcinoma (RCC).
Patients and methods: Patients had advanced non-clear-cell renal carcinoma who underwent 0-1 prior systemic therapies excluding prior immune checkpoint inhibitors. Patients received cabozantinib 40 mg once daily plus nivolumab 240 mg once every 2 weeks or 480 mg once every 4 weeks. Cohort 1 enrolled patients with papillary, unclassified, or translocation-associated RCC; cohort 2 enrolled patients with chromophobe RCC. The primary end point was objective response rate (ORR) by RECIST 1.1; secondary end points included progression-free survival, overall survival, and safety. Next-generation sequencing results were correlated with response.
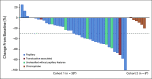
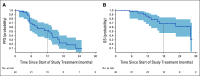
Results: A total of 47 patients were treated with a median follow-up of 13.1 months. Objective response rate for cohort 1 (n = 40) was 47.5% (95% CI, 31.5 to 63.9), with median progression-free survival of 12.5 months (95% CI, 6.3 to 16.4) and median overall survival of 28 months (95% CI, 16.3 to not evaluable). In cohort 2 (n = 7), no responses were observed; one patient had stable disease > 1 year. Grade 3/4 treatment-related adverse events were observed in 32% treated patients. Cabozantinib and nivolumab were discontinued because of toxicity in 13% and 17% of patients, respectively. Common mutations included NF2 and FH in cohort 1 and TP53 and PTEN in cohort 2. Objective responses were seen in 10/12 patients with either NF2 or FH mutations.
Conclusion: Cabozantinib plus nivolumab showed promising efficacy in most non-clear-cell RCC variants tested in this trial, particularly those with prominent papillary features, whereas treatment effects were limited in chromophobe RCC. Genomic findings in non-clear-cell RCC variants warrant further study as predictors of response.
Trial registration: ClinicalTrials.gov NCT03635892.
Conflict of interest statement
Phase II Trial of Cabozantinib Plus Nivolumab in Patients With Non–Clear-Cell Renal Cell Carcinoma and Genomic Correlates
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- American Cancer Society Key statistics about kidney cancer. https://www.cancer.org/cancer/kidney-cancer/about/key-statistics.html
-
- Koh Y, Lim HY, Ahn JH, et al. Phase II trial of everolimus for the treatment of nonclear-cell renal cell carcinoma Ann Oncol 241026–10312013 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

