A TCR mimic monoclonal antibody for the HPV-16 E7-epitope p11-19/HLA-A*02:01 complex
- PMID: 35298559
- PMCID: PMC8929633
- DOI: 10.1371/journal.pone.0265534
A TCR mimic monoclonal antibody for the HPV-16 E7-epitope p11-19/HLA-A*02:01 complex
Abstract
More effective treatments are needed for human papilloma virus (HPV)-induced cancers despite HPV virus vaccination. The oncogenic HPV protein targets are currently undruggable and intracellular and therefore there are no antibodies to these targets. Here we report the discovery of TCR mimic monoclonal antibodies (TCRm mAb) specific for the HPV E7 protein p11-19, YMLDLQPET, when presented on the cell surface in the context of HLA-A*02:01 by use of human phage display libraries. One of the mAbs, 3F8, was able to specifically mediate T cell- redirected cytotoxicity, in a bispecific T cell engager (BiTE) form. While further studies are required to assess the therapeutic potential of this approach, the study provided the proof of concept that TCRm mAb could be a therapeutic strategy for HPV-induced human cancers.
Conflict of interest statement
DAS is on a board of, or has equity in, Lantheus Pharmaceuticals, Sellas, Iovance Biotherapeutics, Pfizer, Actinium Pharmaceuticals, OncoPep, Bridge Medicines, Repertoire, Sapience, and Eureka Therapeutics. TD is a consultant to Eureka Therapeutics. All other authors declare no conflict or competing interests. The authors would like to declare the following patents/patent applications associated with this research: Patents will be filed by Sloan Kettering on behalf of the authors for work described in the paper prior to publication: “Antibodies to HPV peptide MHC complexes.” This does not alter our adherence to PLOS ONE policies on sharing data and materials. No commercial entity has funded this work or any salaries. There are no products in development or marketed products associated with this research. The information listed in our Competing Interests statement does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
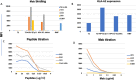
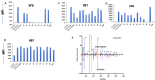

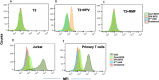
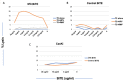
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

