Manufacturing polymeric porous capsules
- PMID: 35298578
- PMCID: PMC8981216
- DOI: 10.1039/d1cc06565c
Manufacturing polymeric porous capsules
Abstract
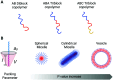
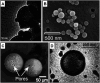
Polymeric porous capsules represent hugely promising systems that allow a size-selective through-shell material exchange with their surroundings. They have vast potential in applications ranging from drug delivery and chemical microreactors to artificial cell science and synthetic biology. Due to their porous core-shell structure, polymeric porous capsules possess an enhanced permeability that enables the exchange of small molecules while retaining larger compounds and macromolecules. The cross-capsule transfer of material is regulated by their pore size cut-off, which depends on the molecular composition and adopted fabrication method. This review outlines the main strategies for manufacturing polymeric porous capsules and provides some practical guidance for designing polymeric capsules with controlled pore size.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Patra J. K. Das G. Fraceto L. F. Campos E. V. R. del M. Rodriguez-Torres P. Acosta-Torres L. S. Diaz-Torres L. A. Grillo R. Swamy M. K. Sharma S. Habtemariam S. Shin H.-S. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnology. 2018;16:71. doi: 10.1186/s12951-018-0392-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

